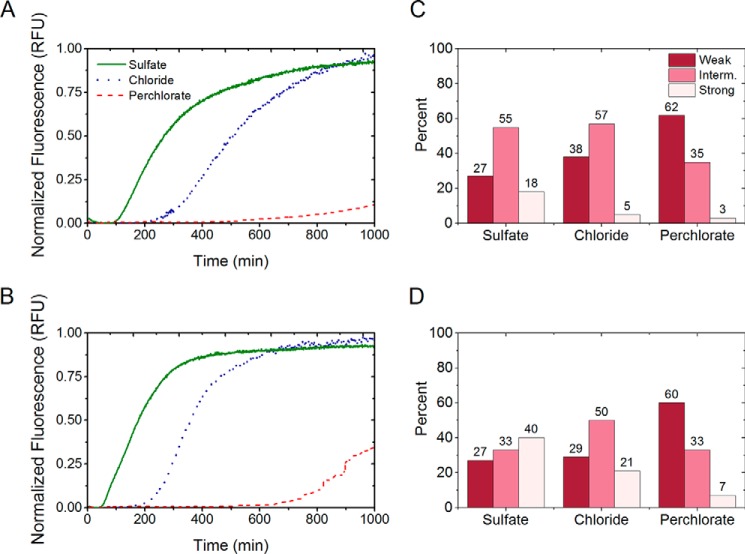
FIGURE 7.
Aggregation profiles of Sup35NMSb and Sup35NMSp in the presence of 0.4 m salts monitored by Thioflavin T fluorescence assay and phenotypic characterization of Sup35NMSb and Sup35NMSp prion strains. A and B, amyloid formation by Sup35NMSb (A) and Sup35NMSp (B) in the presence of 0.4 m sulfate, chloride, or perchlorate showing the effect of Hofmeister ions on their aggregation. In the presence of a strong kosmotrope (sulfate), the lag times are short, and elongation rates are fast. The opposite is true of aggregation in a chaotropic solution (perchlorate), whereas the aggregation plots show intermediate lag times and elongation rates in the presence of the mildly chaotropic chloride ions. RFU, relative fluorescence units. C and D, distribution of weak, intermediate (Interm.), and strong [PSI+] colonies obtained after the transfection of the yeast strains GT987 (expressing Sup35Sb) and GT797 (expressing Sup35Sp) with Sup35NMSb (C) and Sup35NMSp (D) amyloids, respectively, obtained in the presence of sulfate, chloride, or perchlorate salts. For both proteins, amyloids formed in sulfate resulted in a higher number of strong strains that appeared white or faintly pink in color on YPD, and the amyloids formed in perchlorate formed more weak strains appearing dark pink on YPD. Amyloids formed in chloride showed intermediate strain spectra compared with those formed in sulfate and perchlorate.