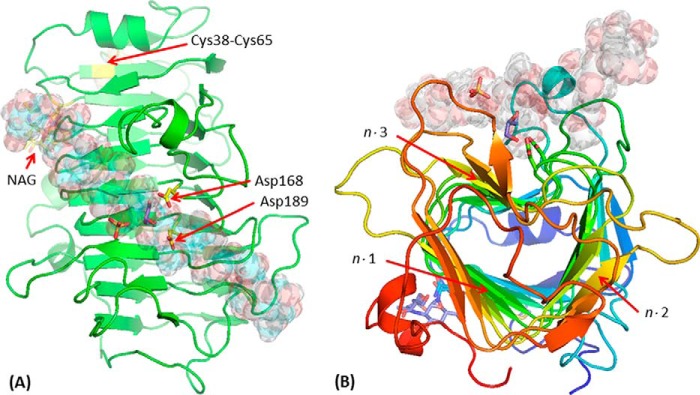
FIGURE 6.
Structure of EndoHf-treated Ani-PME2. The active-site Asp residues (here Asp-168 and Asp-189) are highlighted in a ball-and-stick representation (carbon atoms in yellow); the disordered NAG stub is similarly highlighted (carbon atoms in yellow). Other atoms, including those of a sulfate and a glycerol near the active site, and the disulfide bridge Cys-38–Cys-65, are in standard CPK coloring. A decasaccharide (de-methyl-esterified for residues labeled −5, −4, −3, −2, −1, and +1 and methyl-esterified for residues +2, +3, +4, and +5) is modeled in the binding groove and shown in semi-transparent form. A, view looking down on the active site. Note that the carboxylate group of the decasaccharide residue at the site −1 coincides closely with one of the sulfate anions, and a glycerol is located close to the active-site aspartate residues. B, view looking approximately down the β-helix axis of Ani-PME2, rainbow colored from the N terminus in blue to the C terminus in red. The n·m faces of the β-helix are labeled (helix turn n = 1–12; face m = 1–3).