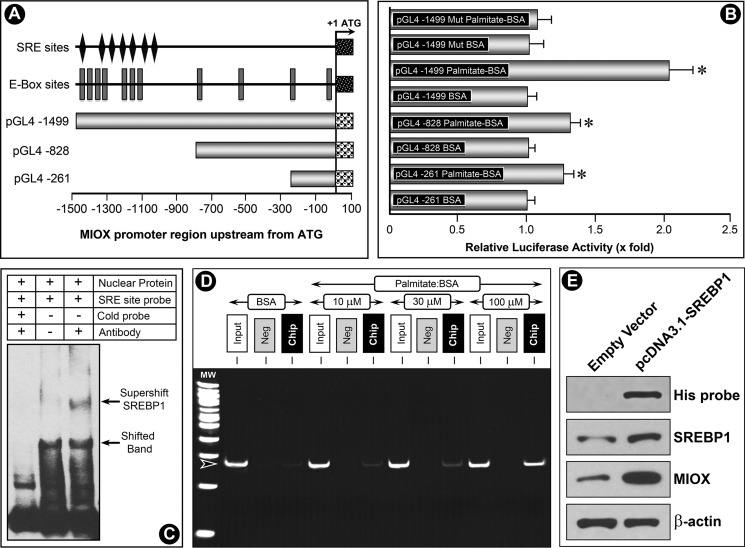
FIGURE 4.
Modulation of miox promoter activity by palmitate/BSA and characterization of binding of Srebp to SRE. Three constructs were generated spanning the miox gene −1500 bp upstream of ORF, and one spanning the entire region had maximum miox basal promoter activity (A). This construct was populated with E-box sites and multiple SRE sites. A mutant construct was generated by modifying the SRE sites by site-directed mutagenesis. The constructs were transfected into HK-2 cells and co-transfected with the luciferase constructs to measure the luciferase activity. Basal promoter activity was designated as 1, and following the palmitate/BSA treatment a variable increase in luciferase activity was observed in all constructs (B). The highest palmitate-induced increase in activity (∼2-fold) was observed in the transfected construct inclusive of E-box and SRE sites. A marginal increase in activity in HK-2 cells transfected with mutant construct was observed, which indicated that the responsiveness of miox promoter to fatty acids is mediated via SRE sites. EMSA revealed a shifted band where a biotin-labeled oligonucleotide probe was included in the incubation mixture (C, lower arrow, middle lane). Unlabeled cold oligonucleotides probe effectively competed because the shifted band disappeared (C, left lane). With inclusion of anti-Srebp1 antibody in the reaction mixture, a super-shifted band to a higher molecular weight was seen (C, upper arrow, right lane). To investigate whether binding of Srebp1 with the miox promoter was dose-dependent, ChIP assays followed by PCR were performed following palmitate/BSA treatment with an expected PCR product size of 271 bp. A dose-dependent increase in the binding of Srebp1 to SRE sites was observed following palmitate/BSA treatment (D). No band was seen in negative control samples where normal rabbit IgG was substituted for Srebp1 antibody. Functionality of Srebp1 in transfected cells was confirmed by Western blot analysis using antibodies directed against His tag, Srebp1, and Miox. His-tagged antibody could confirm the presence of His fusion protein in cells transfected with pcDNA3.1/V5-His TOPO Srebp1, although no band was seen in cells transfected with empty vector (E). The pcDNA-transfected cells also yielded an increased expression of both Srebp1 and Miox, suggesting Srebp1 is functional in transfected cells and can modulate Miox expression (E). These experiments validated that fatty acids up-regulate Srebp1, which then binds to the miox promoter and modulates miox transcription. *, p < 0.01 versus control, n = 4.