FIGURE 3.
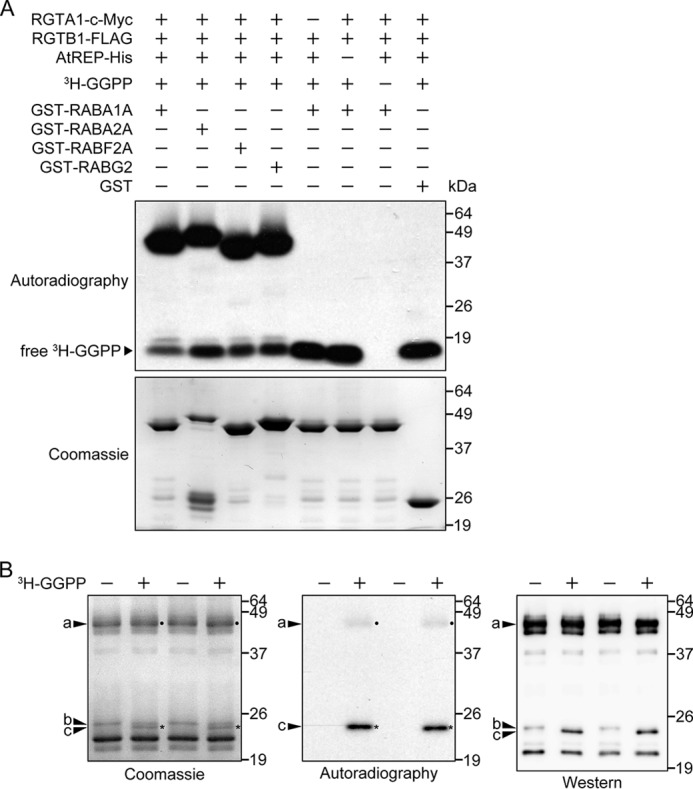
RGTA1·RGTB1 shows Rab-GGT activity in vitro. A, RGTA1·RGTB1 prenylates RABA1A, RABA2A, RABF2A, and RABG2 in vitro, in the presence of AtREP. The presence or absence of individual components in each reaction is indicated by a plus or minus sign, respectively. The reaction mixture was resolved by 10% SDS-PAGE. The x-ray film was exposed to the vacuum-dried SDS-PAGE gel at −80 °C for 48 h to detect radiolabeled 3H-GGPP. The bands of free, unincorporated 3H-GGPP at the bottom of the gels indicate that the labeled lipid substrate was always in excess in the reactions. B, prenylated RABG2 has altered SDS-PAGE migration. In vitro prenylation reactions, including RGTA1·RGTB1, AtREP, FLAG-RABG2, and with (+) or without (−) 3H-GGPP, were carried out and resolved on two 20% SDS-polyacrylamide gels. Proteins on one gel were visualized by Coomassie staining (left), and the gel was subsequently dried and exposed to an x-ray film for 12 h to detect radiolabeled prenylated proteins (center). The corresponding bands are marked with dots or asterisks on their right. Proteins on the other gel were transferred to a nitrocellulose membrane and probed with an anti-FLAG antibody (right). a, GST- and FLAG-double tagged RABG2 (GST-FLAG-RABG2), either prenylated or unprenylated; b, unprenylated FLAG-tagged RABG2 (FLAG-RABG2); c, prenylated FLAG-RABG2 (FLAG-RABG2-GG).
