Abstract
Background
The plant-derived estrogen biochanin A is known to cause vasodilation, but its mechanism of action in hypertension remains unclear. This study was undertaken to investigate the effects and mechanisms of biochanin A on the thoracic aorta in two-kidney, one clip (2K1C) renovascular hypertensive rats.
Methods
Hypertension was induced by clipping the left renal artery, and control age-matched rats were sham treated. Thoracic aortae were mounted in tissue baths to measure isometric tension.
Results
Biochanin A caused concentration-dependent relaxation in aortic rings from 2K1C hypertensive and sham-treated rats, which was greater in 2K1C rats than in sham rats. Biochanin A-induced relaxation was significantly attenuated by removing the endothelium in aortic rings from 2K1C rats, but not in sham rats. Nω-Nitro-l-arginine methyl ester, a nitric oxide synthase inhibitor, or indomethacin, a cyclooxygenase inhibitor, did not affect the biochanin A-induced relaxation in aortic rings from 2K1C and sham rats. By contrast, treatment with glibenclamide, a selective inhibitor of adenosine triphosphate-sensitive K+ channels, or tetraethylammonium, an inhibitor of Ca2+-activated K+ channels, significantly reduced biochanin A-induced relaxation in aortic rings from both groups. However, 4-aminopyridine, a selective inhibitor of voltage-dependent K+ channels, inhibited the relaxation induced by biochanin A in 2K1C rats, whereas no significant differences were observed in sham rats.
Conclusion
These results suggest that the enhanced relaxation caused by biochanin A in aortic rings from hypertensive rats is endothelium dependent. Vascular smooth muscle K+ channels may be involved in biochanin A-induced relaxation in aortae from hypertensive and normotensive rats. In addition, an endothelium-derived activation of voltage-dependent K+ channels contributes, at least in part, to the relaxant effect of biochanin A in renovascular hypertension.
Keywords: Biochanin A, Endothelium, K+ channels, Renovascular hypertension
Introduction
Estrogen replacement therapy markedly reduces the risk of cardiovascular disease in postmenopausal women [1], [2]. However, the use of hormone replacement therapy as a cardioprotective strategy is greatly limited owing to carcinogenic effects in women and feminizing effects in men. Phytoestrogens are naturally occurring plant-derived nonsteroidal estrogens, which also bind to the estrogen receptor, acting as estrogen agonists or antagonists [3], [4].
It has been shown that dietary soy-derived estrogens are vasoactive and have beneficial cardiovascular effects [5]. Both dietary estrogens [6] and estradiol replacement therapy [7] enhance the vasorelaxant response to acetylcholine in female monkeys, whereas systemic arterial compliance was improved by a red clover diet in menopausal women [8]. Biochanin A that naturally occurs in soybeans and clover is estrogen-like [9]. It has been shown to relax the rabbit basilar artery [10] and to induce significant sex-independent relaxation of rabbit coronary arteries [11]. Furthermore, Wang et al [1] subsequently confirmed that biochanin A induces endothelium-independent relaxation in rat aortic rings, of which mechanism involves blockage of Ca2+ entry through the cell membrane and activation of K+ channels. In addition, biochanin A-induced vasorelaxation was increased in spontaneously hypertensive rats than in normotensive rats, which was mediated by the release of endothelium-derived substances that may open voltage-dependent and Ca2+-activated K+ channels in the vascular smooth muscle [12]. Under these backgrounds, we have previously shown that an activation of Ca2+-activated K+ channels in vasorelaxation is altered in two-kidney, one clip (2K1C) renal hypertension [13]. Although an endothelium-derived activation of smooth muscle cell K+ channels, which contributes to biochanin A-induced vasorelaxation, was observed in spontaneously hypertensive rats, the effect of biochanin A on vascular function in 2K1C renovascular hypertension remains unclear.
The present study was designed to examine the mechanisms of the relaxing effects induced by biochanin A in the thoracic aorta isolated from 2K1C renovascular hypertensive rats.
Methods
Induction of 2K1C renovascular hypertension
Renovascular hypertension was induced in rats following the 2K1C Goldblatt model [14]. Briefly, male Sprague–Dawley rats (Samtako Inc., Osan, Korea), weighing 160–180 g, were anesthetized with an intraperitoneal injection of sodium thiopental (40 mg/kg). Under antiseptic conditions, an incision was made on the left flank to access the left renal artery, which was separated from the renal vein and cleaned of connective tissue. A U-shaped solid silver clip with an internal diameter of 0.2 mm was applied to the exposed renal artery, resulting in the partial occlusion of renal perfusion. The contralateral kidney remained untouched, and the wound was closed. A group of age-matched rats were sham treated and served as the control group. The control animals underwent an operation similar to the 2K1C rats except that no clip was used. All animals were fed normal chow and were given tap water. They were used for the experiments at 10 weeks after the clipping, because endothelial dysfunction is associated with the duration of hypertension [15]. Hypertensive rats were selected based on their systolic blood pressure measured in a conscious state by the tail cuff method.
Tissue preparation
The thoracic aorta between the aortic arch and diaphragm was carefully removed and placed in cold, standard physiological salt solution with the following composition: NaCl 118.3mM, KCl 4.7mM, NaHCO3 25mM, MgCl2 1.2mM, KH2PO4 1.2mM, CaCl2 2.5mM, and glucose 11.1mM. Vessels were cleaned of adherent fat and connective tissue, and then cut into 2–3 mm long cylindrical rings under a dissecting microscope. The rings were suspended between two triangle-shaped stainless steel holders in the vessel lumen in organ baths containing 15 mL physiological salt solution maintained at 37±0.05°C, aerated with a mixture of 95% O2 and 5% CO2, and maintained at a pH of 7.4±0.01. One of the holders was fixed at the bottom of the chamber, and the other was connected to a Grass force displacement transducer (FTO3, Quincey, MA, USA) to measure isometric tension development. Prior to initiating specific experimental protocols, the aortic rings were stretched to the point of their optimal length–tension relationship 2 g, which was determined in similar preliminary experiments using repeated exposure to 60mM KCl (obtained by equimolar replacement of NaCl by KCl in the physiological solution), and equilibration for a period of at least 90 minutes.
Protocols
At the beginning of the experiment, aortic rings were stimulated with 60mM KCl to test their functional integrity. In all experiments, aortic rings from 2K1C and sham-operated rats were precontracted to 50% effective concentration (EC50) with phenylephrine (3×10−7M in sham and 4×10−8M in 2K1C), which were obtained in preliminary experiments. When the contractile response achieved a steady state, relaxation–response curves to the cumulative addition of biochanin A (from 10−7M to 10−4M), acetylcholine (from 10−9M to 10−5M), or sodium nitroprusside (SNP; from 10−10M to 10−6.5M) were determined. In an alternate set of experiments, the aorta was pretreated for 10 minutes with biochanin A (3×10−5M) prior to the addition of phenylephrine in the case of acetylcholine- and SNP-induced relaxation. To verify the role of functional endothelium in the vascular relaxant effects of biochanin A, the endothelium was removed from some thoracic aortae by gently rubbing the intimal surface with a moistened cotton swab. The successful removal of the endothelial cells from aortic rings was confirmed via the inability of acetylcholine to induce relaxation.
In another experiments, the nitric oxide synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 10−4M) or the cyclo-oxygenase inhibitor indomethacin (10−5M), and K+ channel blockers glibenclamide (3×10−6M), tetraethylammonium (TEA, 10−3M), or 4-aminopyridine (10−3M), were added to the bath 10 minutes prior to the addition of phenylephrine.
Drugs
Acetylcholine, 4-aminopyridine, biochanin A, glibenclamide, indomethacin, l-NAME, SNP, and TEA were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals were of analytical grade. Biochanin A, glibenclamide, and indomethacin were dissolved in dimethylsulfoxide, and the others were prepared in distilled water. The final concentrations of dimethylsulfoxide were <0.05%, which did not alter the contraction or relaxation responses.
Statistical analysis
The values presented in the figures are the means and standard errors of the means. Relaxant responses are presented as the percent change in phenylephrine-induced contractile tension. The concentration causing half-maximal relaxation (IC50) was determined by plotting the percentage of responses and expressed as the mean of negative log molar (pD2) for individual tissue response, using the Origin software (Northampton, MA, USA). Statistical comparisons were performed using Student t test or analysis of variance followed by Duncan’s test for multiple comparisons. A P value<0.05 was considered statistically significant.
Results
The systolic blood pressure was 190±5 mmHg (n=44, P<0.05) and 137±4 mmHg (n=40) in 2K1C hypertensive and sham-clipped rats, respectively.
Vasorelaxant responses to biochanin A
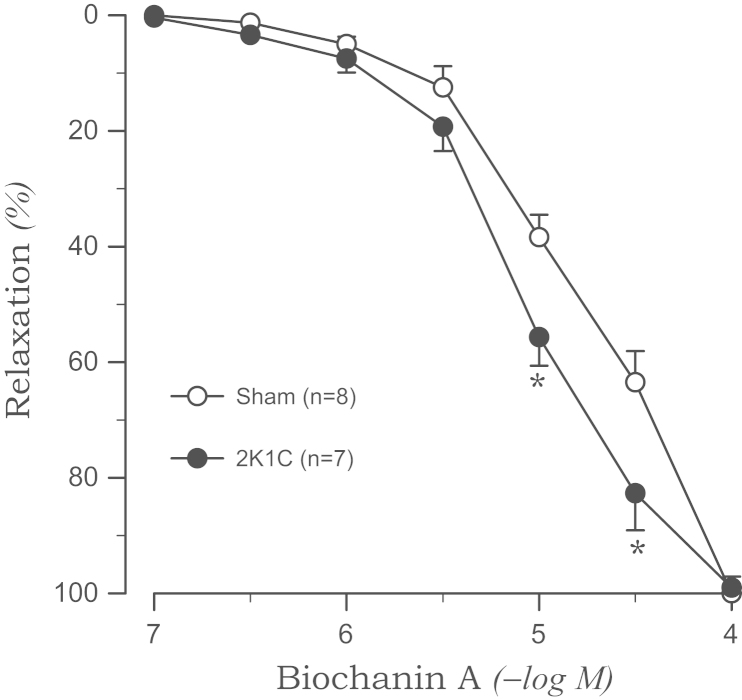
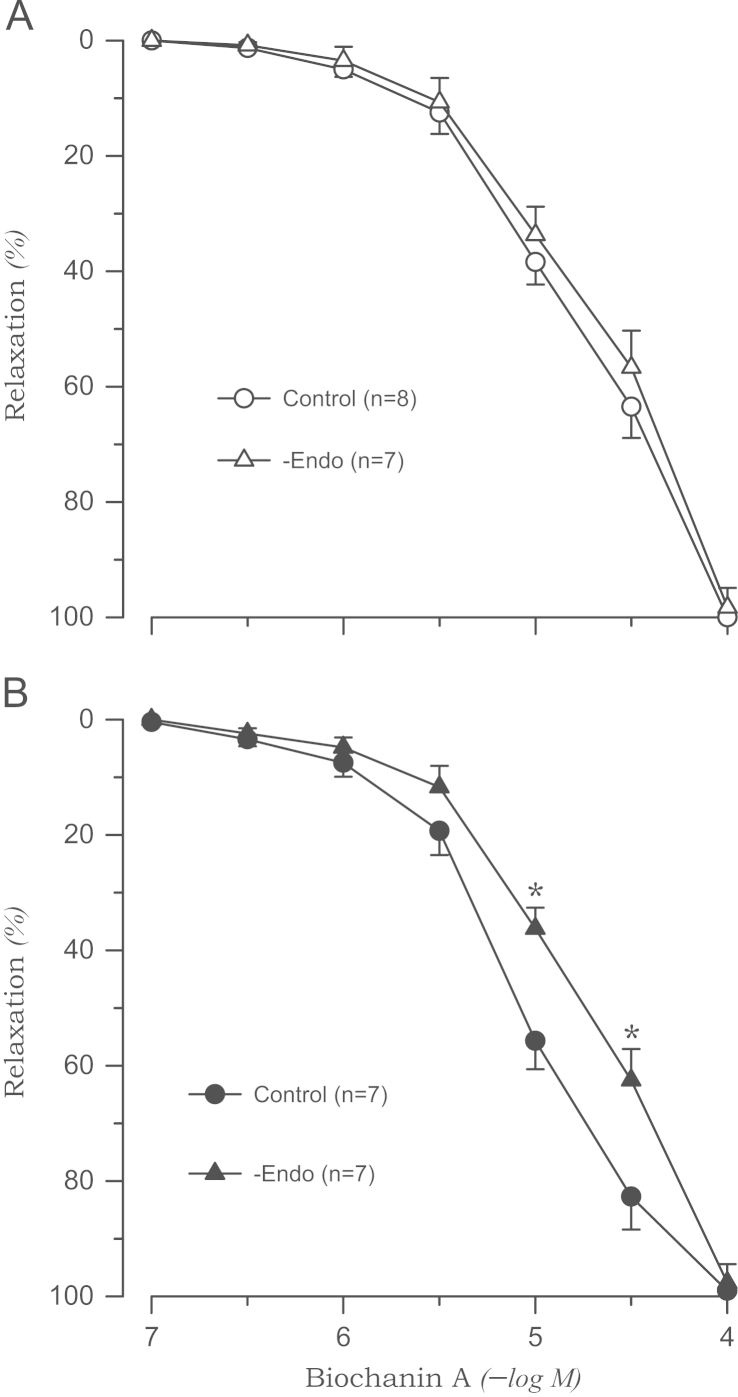
The tension induced by phenylephrine was enhanced in the aortic rings from 2K1C rats (1.45±0.07 g, P<0.05) compared with those from sham rats (1.12±0.06 g). Biochanin A completely relaxed the aortic rings from 2K1C and sham rats when they were precontracted with phenylephrine. However, the relaxation was augmented in the aortic rings from 2K1C rats than in the rings from sham rats (pD2: 5.05±0.08 vs. 4.67±0.07, P<0.05). This effect was more pronounced at the higher concentration of biochanin A (Fig. 1). Biochanin A-induced relaxation was significantly attenuated by the removal of the endothelium in the aortic rings from 2K1C rats (pD2: 4.69±0.06, P<0.05), whereas no significant differences were observed in rings from sham rats (Fig. 2).
Figure 1.
Biochanin A-induced vasorelaxation in phenylephrine-precontracted aortic rings from sham-clipped control and 2K1C hypertensive rats. Points represent means±SE for the number (n) of experiments in parentheses. ⁎P<0.05, compared with sham values. 2K1C, two-kidney, one clip.
Figure 2.
Biochanin A-induced vasorelaxation in phenylephrine-precontracted aortic rings without endothelium from sham-clipped control (A) and 2K1C hypertensive rats (B). ⁎P<0.05, compared with corresponding control values. 2K1C, two-kidney, one clip; −Endo, without endothelium.
Vasorelaxant responses to acetylcholine and SNP
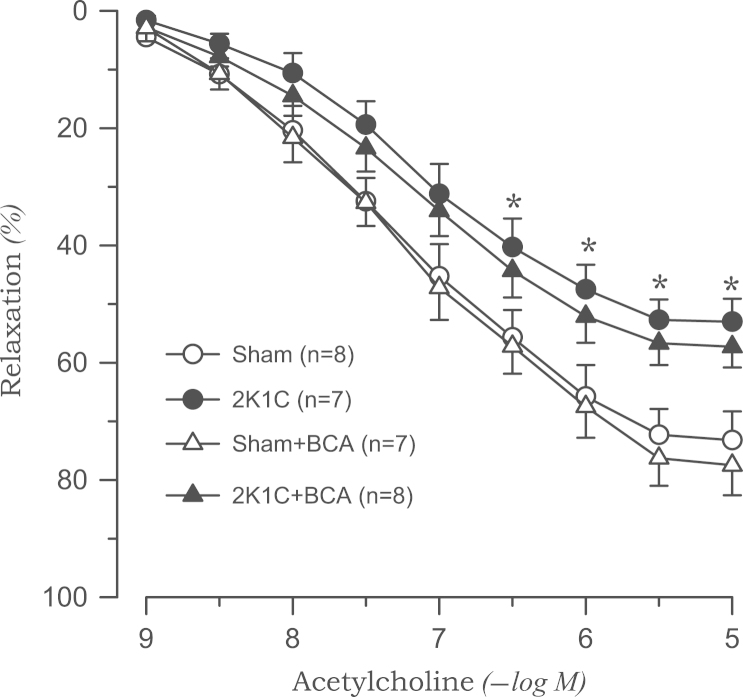
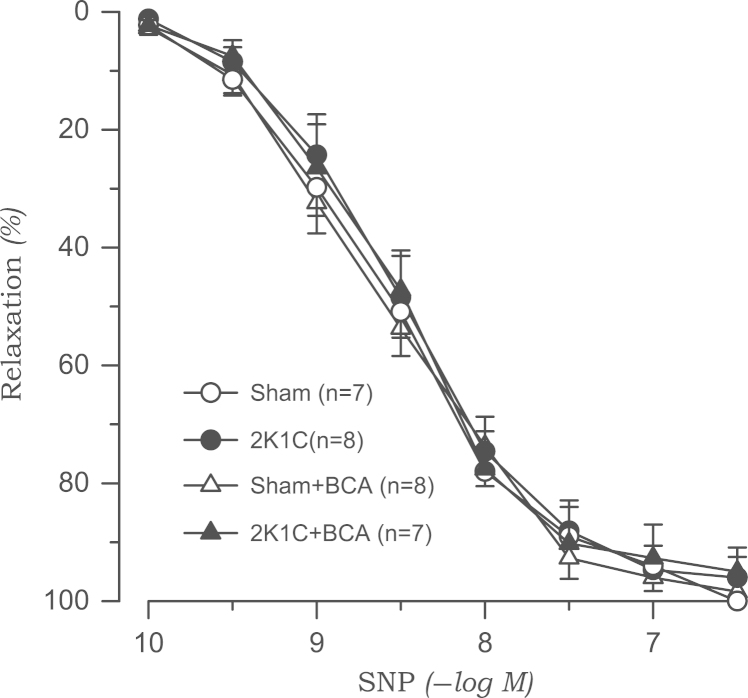
Acetylcholine-induced vasodilation was significantly attenuated in the aortic rings from 2K1C rats than in those from sham rats. Treatment with l-NAME (10−4M) completely blocked the acetylcholine-induced vasodilatory effect in both 2K1C and sham groups (data not shown). Biochanin A (3×10−5M) had no effect on acetylcholine-induced vasodilation in either sham or 2K1C rats (Fig. 3). The vasorelaxation response to SNP was not altered in 2K1C rats. Biochanin A did not affect SNP-induced vasodilation (Fig. 4).
Figure 3.
Effects of biochanin A on vasodilatory responses induced by acetylcholine in aortic rings from sham-clipped control and 2K1C hypertensive rats. ⁎P<0.05, compared with corresponding sham values. 2K1C, two-kidney, one clip; BCA, biochanin A.
Figure 4.
Effects of biochanin A on vasodilatory responses induced by sodium nitroprusside in aortic rings from sham-clipped control and 2K1C hypertensive rats. 2K1C, two-kidney, one clip; BCA, biochanin A; SNP, sodium nitroprusside.
Effects of l-NAME and indomethacin on biochanin A-induced vasorelaxation
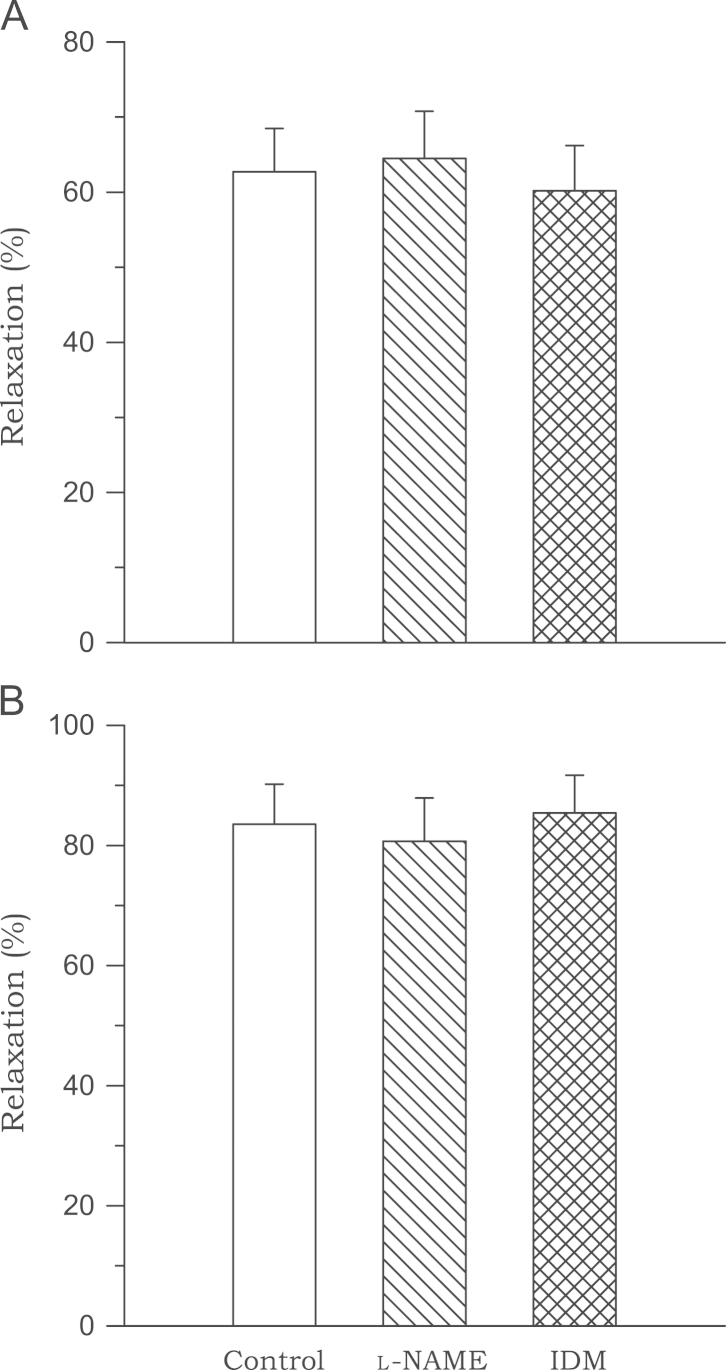
Preincubation with l-NAME or indomethacin had no effect on biochanin A (3×10−5M)-induced vasodilation in either sham or 2K1C rats (Fig. 5).
Figure 5.
Effects of Nω-nitro-l-arginine methyl ester and indomethacin on the relaxation induced by biochanin A in aortic rings from sham-clipped control (A) and 2K1C hypertensive (B) rats. Data are obtained from six to eight experiments. 2K1C, two-kidney, one clip; IDM, indomethacin; l-NAME, Nω-nitro-l-arginine methyl ester.
Effects of K+channel blockers on biochanin A-induced vasorelaxation
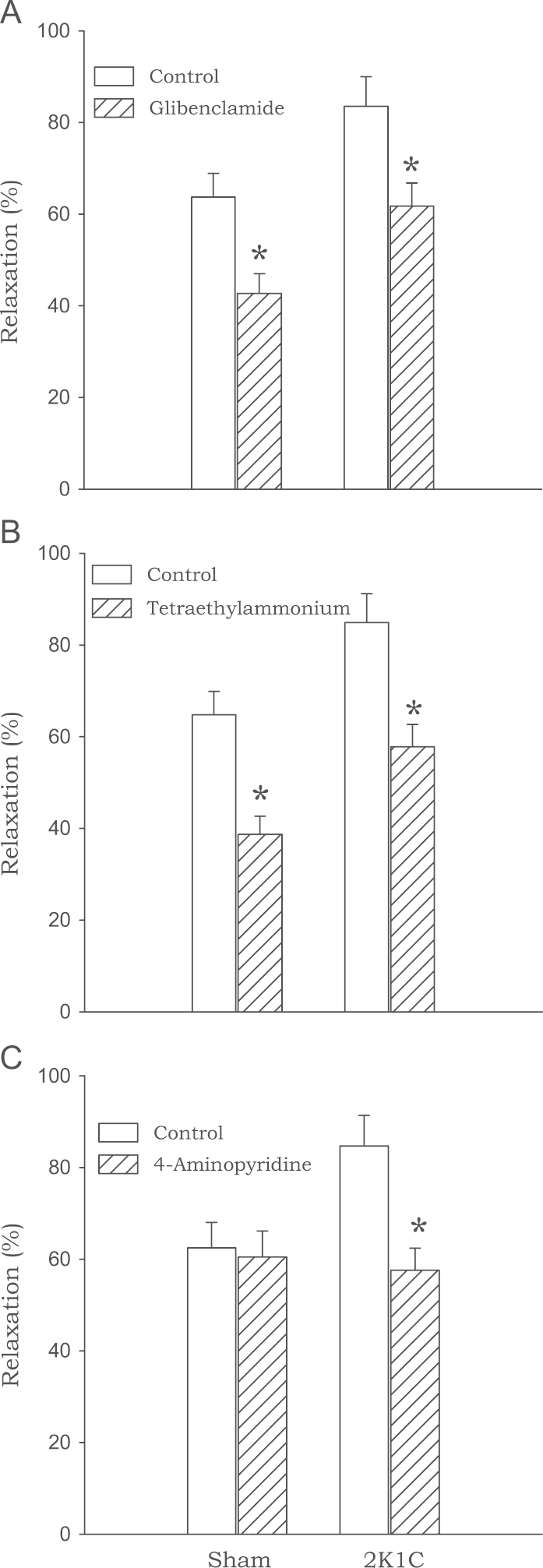
Pretreatment with glibenclamide, an inhibitor of adenosine triphosphate (ATP)-sensitive K+ channels, and TEA, an inhibitor of Ca2+-activated K+ channels, significantly reduced biochanin A (3×10−5M)-induced relaxation in 2K1C and sham-clipped rats. However, 4-aminopyridine, an inhibitor of voltage-dependent K+ channels, inhibited biochanin A-induced relaxation only in 2K1C rats with no effects in sham rats (Fig. 6).
Figure 6.
Effects of glibenclamide (A), tetraethylammonium (B), and 4-aminopyridine (C) on the relaxation induced by biochanin A in aortic rings from sham-clipped control and 2K1C hypertensive rats. Data are obtained from six to eight experiments. ⁎P<0.05, compared with corresponding control values. 2K1C, two-kidney, one clip.
Discussion
We previously confirmed that vascular reactivity to contractile agonist is enhanced in disease states such as hypertension [16]. As shown in our previous findings, the contractile response to phenylephrine was augmented in 2K1C hypertensive rats than in sham-clipped normotensive rats. In the present study, biochanin A induced dose-dependent relaxation in phenylephrine-precontracted aortic rings with intact endothelium isolated from 2K1C and sham rats, of which the magnitude was augmented in hypertensive rats. The relaxant effect of biochanin A in aortic rings from sham rats with or without endothelium was comparable, suggesting that biochanin A-induced relaxation is endothelium-independent and may directly affect the vascular smooth muscle in normotensive rats. Similar results have been demonstrated in rabbit coronary [11] or basilar arteries [10] and in rat aortae [12]. However, as has been shown previously in genetically hypertensive rats [12], the relaxant effect of biochanin A was significantly attenuated by the removal of the endothelium in aortic rings from 2K1C hypertensive rats. These observations imply that the vasorelaxing effect of biochanin A is partially endothelium dependent in hypertensive aortae, whereas it is endothelium independent in sham rats.
Acetylcholine causes NO release via the activation of specific endothelial receptors, resulting in the activation of endothelial NOS [17]. The present study confirmed earlier observations [15], [18] that endothelium-dependent relaxation to acetylcholine is markedly depressed in 2K1C hypertensive rats compared with that in sham-clipped control rats. Treatment with l-NAME completely inhibited the acetylcholine-induced vasodilatory effect in both groups, suggesting that acetylcholine-induced vasodilation is largely attributable to NOS-derived NO. Although the relaxation induced by acetylcholine was attenuated in hypertensive rats, biochanin A did not affect acetylcholine-induced endothelium-dependent vasorelaxation in either 2K1C or sham rats. In addition, biochanin A had no effect on SNP (which is an NO donor)-induced endothelium-independent vasodilation in aortic rings from 2K1C and sham rats. These results indicate that the functions of NO synthesis or NO-mediated relaxation in aortic rings from hypertensive and normotensive rats are not altered by biochanin A.
Although biochanin A did not affect acetylcholine-induced vasorelaxation in 2K1C hypertensive and sham rats in our experiments, in order to clarify whether the vasodilator NO is involved in biochanin A-induced relaxation, the effects of l-NAME on the relaxation induced by biochanin A were examined. l-NAME did not affect biochanin A-induced relaxation in 2K1C and sham rats. The results again imply that the role of NO is not involved in biochanin A-induced relaxation in either group. The results of the present study are in agreement with previous data from the coronary artery11 and aorta,12 which suggest that vasodilator NO is not a candidate contributor to biochanin A-induced relaxation. Conversely, Torregrosa et al [10] reported that biochanin A-induced relaxation is partially reduced by l-NAME treatment, which indicates that the relaxant effect of biochanin A in aortae with endothelium from rabbits is due to the release of NO. One possible explanation may be attributed to the differences in the experimental conditions, species, or strains [12]. Alternatively, the endothelial release of prostacyclins or endothelium-derived hyperpolarizing factor (EDHF) other than NO may be responsible [19]. In the present study, however, indomethacin, an inhibitor of the synthesis of prostaglandins [20], had little effect on biochanin A-induced relaxation in aortic rings from 2K1C and sham rats. Taken together, an involvement of an EDHF, acting through K+ channels to cause hyperpolarization of vascular smooth muscle cells [21], may be raised. It has been indeed known that the EDHF-mediated vasorelaxation was preserved in an animal model of renovascular hypertension [22], [23].
The EDHF passes through the internal elastic lamina and vascular smooth muscle, and causes an activation of ion channels, thus initiating smooth muscle hyperpolarization and inducing relaxation [24]. In the endothelium and smooth muscle, K+ channels control the vascular tone and arterial blood pressure [25]. The activation of K+ channels causes a hyperpolarization of cell membranes in vascular smooth muscle and thus inhibits Ca2+ influx via voltage-gated Ca2+ channels. Therefore, an activation of K+ channels may play a role in Ca2+ signaling and modulate the arterial tone [26]. There are several types of K+ channels preserved in vascular smooth muscle [27]. In the present study, to examine whether the K+ channels contribute to biochanin A-induced vasodilation in vascular smooth muscle, we pretreated with the K+ channel blockers glibenclamide, TEA, and 4-aminopyridine in endothelium-intact aortic rings from 2K1C hypertensive and sham rats. Biochanin A-induced relaxation was significantly reduced by glibenclamide, a blocker of ATP-sensitive K+ channels, in 2K1C and sham rats. In addition, TEA, which blocks large-conductance Ca2+-activated K+ channels when used in appropriate concentrations [12], also inhibited the relaxant effect of biochanin A in both groups. These results imply that activation of both ATP-sensitive and Ca2+-activated K+ channels may be involved in biochanin A-induced relaxation in hypertensive and normotensive rats. Interestingly, 4-aminopyridine, an inhibitor of voltage-dependent K+ channels, significantly attenuated biochanin A-induced relaxation in aortic rings from 2K1C rats, but no significant differences were observed in sham rats. Therefore, the greater relaxation induced by biochanin A in rings from 2K1C rats compared with those from sham rats in our experiment, may in part be responsible for the voltage-dependent K+ channels and evoke hyperpolarization of the smooth muscle cells. Similar results were also observed in genetically hypertensive rats, in which the activation of K+ channels contributes to the vasorelaxation in hypertension [12]. In addition, a previous study reported that TEA inhibits the vasorelaxant effect of biochanin A in normotensive rats [12], whereas the effects of TEA on biochanin A-induced relaxation were comparable between 2K1C and sham rats in this study. A possible discrepancy may be attributed to the different animal species used in this study.
In summary, the relaxant effect of biochanin A is augmented in aortae of 2K1C hypertensive rats compared with those of sham-clipped control rats, and the relaxation is endothelium-independent in sham rats, whereas it is both endothelium-dependent and -independent in 2K1C rats. An activation of vascular smooth muscle K+ channels may be involved in biochanin A-induced relaxation in both 2K1C and sham rats. In addition, the enhanced relaxation by biochanin A in renovascular hypertension is mediated by endothelium-derived substances that may evoke an activation of voltage-dependent K+ channels in vascular smooth muscle.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
This study was supported by research funds from Chosun University, Gwangju, Korea in 2013.
References
- 1.Wang HP, Mei RH, Li XY, Zhao MH, Lu Y, Xia Q, Bruce I. Endothelium-independent vasorelaxant effect of the phyto-estrogen biochanin A on rat thoracic aorta. Conf Proc IEEE Eng Med Biol Soc. 2005;3:2244–2247. doi: 10.1109/IEMBS.2005.1616910. [DOI] [PubMed] [Google Scholar]
- 2.Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:133–138. doi: 10.1097/MNH.0b013e3283431921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belcher SM, Zsarnovszky A. Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular mechanisms. J Pharmacol Exp Ther. 2001;299:408–414. [PubMed] [Google Scholar]
- 4.Sumien N, Chaudhari K, Sidhu A, Forster MJ. Does phytoestrogen supplementation affect cognition differentially in males and females? Brain Res. 2013;1514:123–127. doi: 10.1016/j.brainres.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony MS, Clarkson TB, Hughes Jr CL, Morgan TM, Burke GL:Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system of peripubertal rhesus monkeys. J Nutr 126:43–50, 1996 [DOI] [PubMed]
- 6.Honore EK, Williams JK, Anthony MS, Clarkson TB. Soy isoflavones enhance coronary vascular reactivity in atherosclerotic female macaques. Fertil Steril. 1997;67:148–154. doi: 10.1016/s0015-0282(97)81872-9. [DOI] [PubMed] [Google Scholar]
- 7.Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81:1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- 8.Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness: a placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23:1066–1071. doi: 10.1161/01.ATV.0000072967.97296.4A. [DOI] [PubMed] [Google Scholar]
- 9.Szliszka E, Czuba ZP, Mertas A, Paradysz A, Krol W. The dietary isoflavone biochanin-a sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urol Oncol. 2013;31:331–342. doi: 10.1016/j.urolonc.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Torregrosa G, Burguete MC, Perez-Asensio FJ, Salom JB, Gil JV, Alborch E. Pharmacological profile of phytoestrogens in cerebral vessels: in vitro study with rabbit basilar artery. Eur J Pharmacol. 2003;482:227–234. doi: 10.1016/j.ejphar.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Figtree GA, Griffiths H, Lu YQ, Webb CM, MacLeod K, Collins P. Plant-derived estrogens relax coronary arteries in vitro by a calcium antagonistic mechanism. J Am Coll Cardiol. 2000;35:1977–1985. doi: 10.1016/s0735-1097(00)00645-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang HP, Gao Q, Mei RH, Zhao MH, Lu Y, Li XY, Bruce IC, Xia Q. Mechanisms underlying biochanin A-induced relaxation of the aorta differ between normotensive and hypertensive rats. Clin Exp Pharmacol Physiol. 2006;33:802–807. doi: 10.1111/j.1440-1681.2006.04443.x. [DOI] [PubMed] [Google Scholar]
- 13.Choi S, Kim H, Jun JY, Yoon PJ, Kim HL, Chung JH, Yeum CH. Role of KCa channels in SNAP-induced relaxation of aorta from renal hypertensive rats. Kor J Nephrol. 2007;26:398–403. [Google Scholar]
- 14.Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension, I: the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigmon DH, Beierwaltes WH. Influence of nitric oxide in the chronic phase of two-kidney one clip renovascular hypertension. Hypertension. 1998;31:649–656. doi: 10.1161/01.hyp.31.2.649. [DOI] [PubMed] [Google Scholar]
- 16.Choi S, Kim HI, Park SH, Lee MJ, Jun JY, Kim HL, Chung JH, Yeum CH. Endothelium-dependent vasodilation by ferulic acid in aorta from chronic renal hypertensive rats. Kor J Nephrol. 2012;31:227–233. doi: 10.1016/j.krcp.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forstermann U, Nakane M, Tracey WR, Pollock JS. Isoform of nitric oxide synthase: functions in the cardiovascular system. Eur Heart J. 1993;14:10–15. [PubMed] [Google Scholar]
- 18.Callera GE, Varanda WA, Bendhack LM. Impaired relaxation to acetylcholine in 2K-1C hypertensive rat aortas involves an abnormal contribution of endothelial factors. Gen Pharmacol. 2000;34:379–389. doi: 10.1016/s0306-3623(01)00075-1. [DOI] [PubMed] [Google Scholar]
- 19.Dal-Ros S, Bronner C, Schott C, Kane MO, Chataigneau M, Schini-Kerth VB, Chataigneau T. Angiotensin II-induced hypertension is associated with a selective inhibition of endothelium-derived hyperpolarizing factor-mediated responses in the rat mesenteric artery. J Pharmacol Exp Ther. 2009;328:478–486. doi: 10.1124/jpet.108.145326. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira SH, Moncada S, Vane JR. Further experiments to establish that the analgesic action of aspirin-like drugs depends on the inhibition of prostaglandin biosynthesis. Br J Pharmacol. 1973;47:629–630. [PMC free article] [PubMed] [Google Scholar]
- 21.Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 22.Sendão Oliveira AP, Bendhack LM. Relaxation induced by acetylcholine involves endothelium-derived hyperpolarizing factor in 2-kidney 1-clip hypertensive rat carotid arteries. Pharmacology. 2004;72:231–239. doi: 10.1159/000080378. [DOI] [PubMed] [Google Scholar]
- 23.Christensen FH, Stankevicius E, Hansen T, Jørgensen MM, Valverde VL, Simonsen U, Buus NH. Flow- and acetylcholine-induced dilatation in small arteries from rats with renovascular hypertension—effect of tempol treatment. Eur J Pharmacol. 2007;566:160–166. doi: 10.1016/j.ejphar.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 24.Luksha L, Agewall S, Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009;202:330–344. doi: 10.1016/j.atherosclerosis.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 26.Köhler R, Ruth P. Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice. Pflugers Arch-Eur J Physiol. 2010;459:969–976. doi: 10.1007/s00424-010-0819-z. [DOI] [PubMed] [Google Scholar]
- 27.Nelson MT, Quayleand JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]