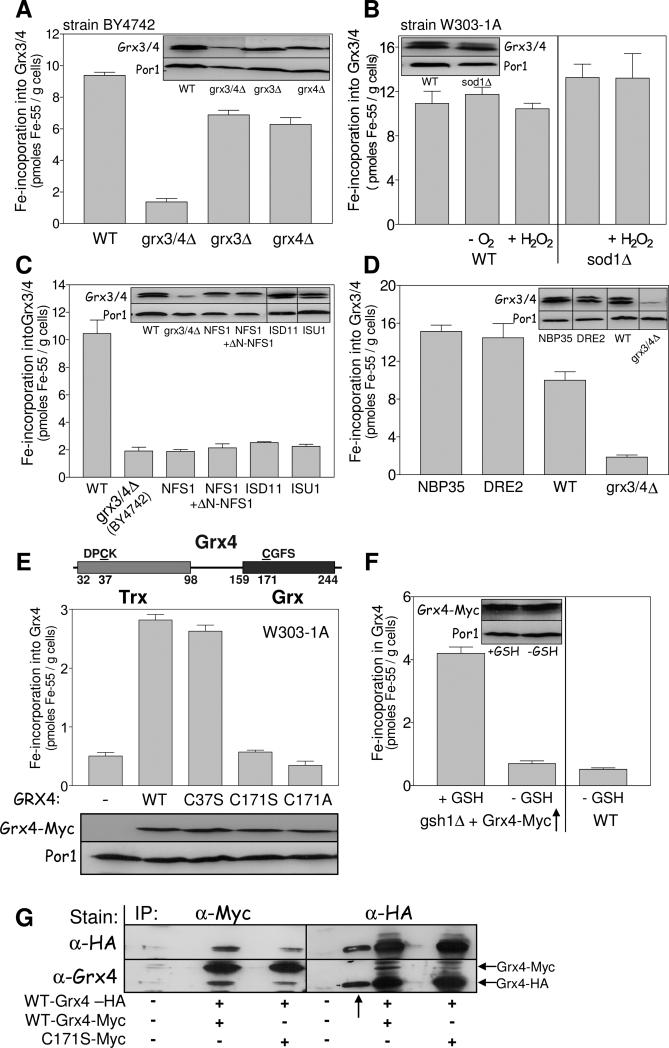
Fig. 5. Grx3/4 assemble a bridging Fe/S cluster independently of the CIA machinery.
(A) 55Fe binding to Grx3/4 was determined in wild-type (WT), grx3Δ, grx4Δ and grx3/4Δ cells (strain background BY4742). Grx3/4 protein levels were assessed by immunostaining. Por1 served as a loading control. The anti-Grx4 antiserum recognizes an unspecific band, in addition to Grx3/4. (B) 55Fe binding to Grx3/4 was determined in WT and sod1Δ cells (strain background W303-1A). Where indicated, cells were pretreated with 1 mM H2O2 for 10 min or examined under anaerobic (−O2) conditions. (C, D) 55Fe binding to Grx3/4 was determined in WT, Gal-NFS1 (with and without a plasmid expressing a cytosolic version of Nfs1 (+ΔN-NFS1)), Gal-ISD11, Gal-ISU1 (C) or Gal-NBP35 and Gal-DRE2 cells (D) after growth in SD medium for 40 h. BY4742 grx3/4Δ cells served as a background control. Grx3/4 and Por1 levels were assessed by immunostaining. (E) Top: Schematic presentation of the domain structure of Grx4. Middle: C-terminally Myc-tagged WT, C37S, C171S, or C171A mutant Grx4 were expressed in WT cells from vector pCM189, and 55Fe binding to Grx4 was determined by radiolabelling and immunoprecipitation with α-Myc beads. Bottom: Grx4-Myc and Por1 levels were assessed by immunostaining. (F) WT and gsh1Δ cells overproducing Grx4-Myc were cultivated in the presence or absence of 1 mM GSH for 64 h (Sipos et al., 2002). 55Fe binding to Grx4-Myc was determined by immunoprecipitation with α-Myc beads. (G) Extracts of WT cells expressing C-terminally HA-tagged Grx4 and wild-type or C171S Grx4-Myc as indicated were subjected to immunoprecipitation (IP) with α-Myc or α-HA-antibodies. The precipitate was analyzed by immunostaining with α-HA or α-Grx4 antibodies. The arrow (↑) indicates a lane with a molecular mass marker which is stained by the α-HA antiserum.