Abstract
Objectives
IPX066 is an oral, extended-release capsule formulation of carbidopa-levodopa (CD-LD) available in 4 strengths. The goals of this investigation were to assess the dose proportionality of IPX066 and to study the effects of a high-fat, high-calorie meal and of sprinkling the capsule contents on applesauce on the pharmacokinetics of IPX066 in healthy volunteers.
Methods
Three open-label studies were conducted. In the first study, subjects received 1 capsule of each IPX066 strength (23.75–95, 36.25–145, 48.75–195, and 61.25–245 mg of CD-LD). In the second study, subjects received 1 and 2 capsules of IPX066 245-mg LD under fasting conditions. In the third study, subjects received 2 capsules of IPX066 245-mg LD under 3 conditions: fasting; following a high-fat, high-calorie breakfast; and with the capsule contents sprinkled on applesauce under fasting conditions.
Results
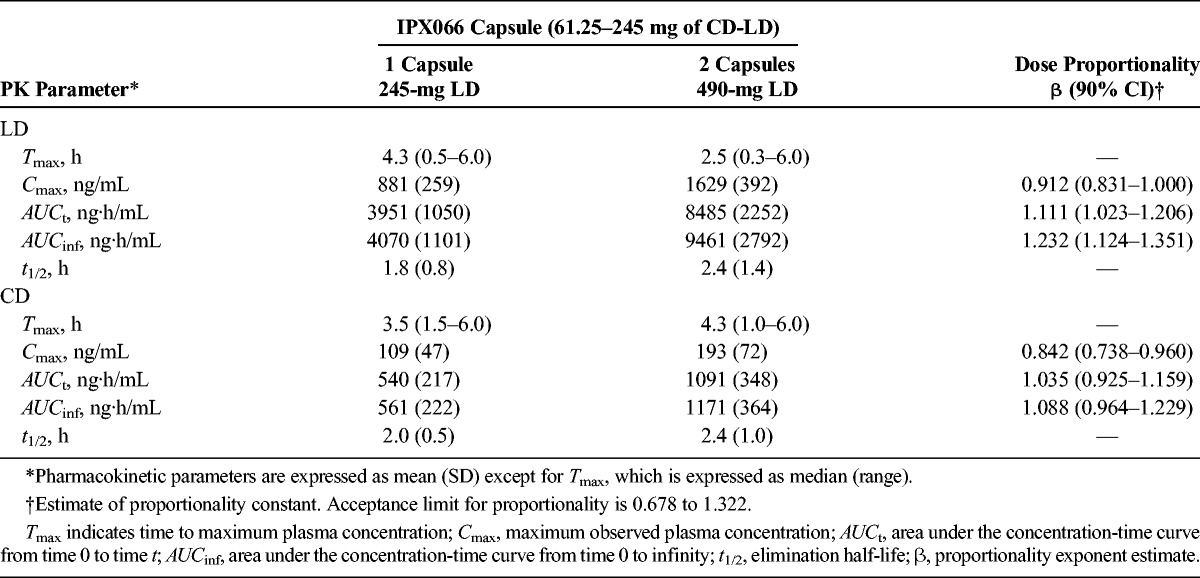
Peak plasma concentrations (Cmax) and systemic exposure (AUCt, AUCinf) for LD and CD increased dose-proportionally over the range of the IPX066 capsule strengths. Comparison of 1 and 2 IPX066 245-mg LD capsules showed dose-proportional pharmacokinetics for Cmax and AUCt. Sprinkling the capsule contents on applesauce did not affect the pharmacokinetics. A high-fat, high-calorie meal delayed the initial increase in LD concentration by approximately 1 to 2 hours, reduced Cmax by 21%, and increased AUCinf by 13% compared with the fasted state.
Conclusions
IPX066 shows dose-proportional pharmacokinetics. Sprinkling the capsule contents on applesauce does not affect the pharmacokinetics; a high-fat, high-calorie meal delayed absorption by 1 to 2 hours, slightly reduced Cmax, and slightly increased extent of absorption.
Key Words: IPX066, levodopa, pharmacokinetics, dose proportionality, effect of food
Levodopa (LD) combined with a peripheral inhibitor of aromatic L-amino acid decarboxylase, such as carbidopa (CD), continues to be the most effective treatment of Parkinson disease and is well tolerated at all stages of the disease.1–3 Immediate-release formulations of LD require frequent dosing because of the short half-life of LD and result in marked fluctuations in the LD plasma concentrations. The pulsatile LD profile results in fluctuations in the clinical response such as on-off and wearing off and may play an important role in LD-induced dyskinesia.4,5 Intravenous and intraduodenal infusion of LD provide more stable plasma concentrations and can result in a smoother clinical response in patients with motor fluctuations.6–8 However, intravenous infusion is not practical for long-term therapy, and duodenal infusion requires surgery and may only be appropriate for the most severe patients. An oral product that provides more consistent LD absorption and more stable plasma concentrations would address a major unmet clinical need to enhance efficacy and reduce or prevent motor oscillations and drug-induced dyskinesias.9 However, current sustained-release formulations of CD-LD and the addition of a cathechol-O-methyltransferase inhibitor (eg, entacapone) to immediate-release CD-LD have been of limited value.10,11 Controlled-release formulations have been associated with erratic absorption, variable plasma concentrations, and a delayed onset of effect.12,13 Carbidopa-LD products containing entacapone have been associated with a shorter time to onset of dyskinesia and increased frequency of dyskinesia compared with CD-LD products.14
IPX066 (RYTARY [CD and LD]; Impax Laboratories, Inc, Hayward, Calif) is an extended-release multiparticulate capsule formulation of CD-LD that has demonstrated efficacy in patients with early and advanced Parkinson disease.15,16 IPX066 capsules are available in a 1:4 ratio of CD:LD in 4 CD-LD dose strengths: 23.75–95, 36.25–145, 48.75–195, and 61.25–245 mg of CD-LD.
Food has been reported to affect the absorption of various drugs and, in particular, affect the performance of LD formulations.17–19 Absorption of LD occurs mainly in the proximal third of the small intestine. Delayed gastrointestinal transit may affect CD-LD absorption. Gastric emptying is affected by the composition and physicochemical properties of a meal. In addition, LD and its derivative dopamine have also been reported to affect gastric emptying.20,21
The objectives for this investigation were to characterize the dose proportionality of the 4 dose strengths of IPX066 capsules and to compare the pharmacokinetics of 1 and 2 capsules of the highest strength (61.25-245 mg of CD-LD). In addition, the effect of a high-fat, high-calorie breakfast and the effect of sprinkling the capsule contents on applesauce on the pharmacokinetics of IPX066 were assessed.
MATERIALS AND METHODS
Three studies were conducted at a single clinical center (Cetero Research, St Charles, Mo). Protocols were approved by the institutional review board (IRB), and the studies were conducted in accordance with International Conference of Harmonization Guidelines including the ethical principles of the Declaration of Helsinki on biomedical research involving human subjects and IRB policies. Before participation, each subject was required to read and sign an IRB-approved consent form explaining the nature, purpose, and possible risks and benefits of the study and the duration of an individual's participation.
Subjects
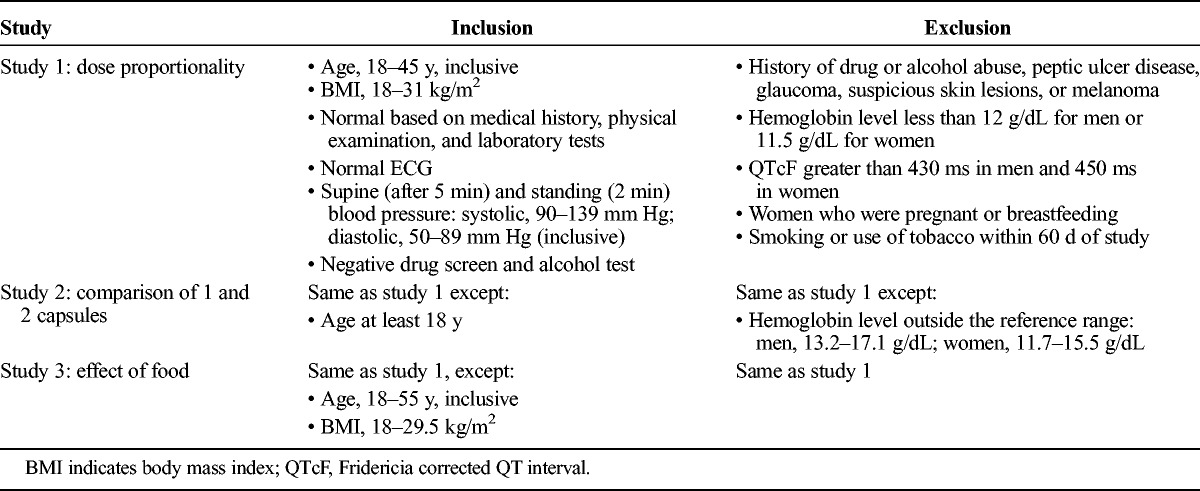
Healthy male and female subjects (aged 18-53 years, inclusive) were enrolled in the 3 studies. Key inclusion and exclusion criteria for each study are outlined in Table 1. In each of the studies, subjects were required to use a medically acceptable method of contraception. In addition, use of alcohol, grapefruit juice, or products containing Seville oranges was prohibited beginning 72 hours before dosing until the end of the study, and subjects were not allowed to smoke or use tobacco products within 60 days before the first dose until the end of the study. Use of prescription medications (except contraceptives), over-the-counter medications (except acetaminophen and daily multivitamins), and herbal products was prohibited 14 days before dosing (21 days for monoamine oxidase inhibitors) until the end of the study.
TABLE 1.
Key Inclusion and Exclusion Criteria Across Studies
Study Design
Study 1 was a randomized, single-dose, crossover study to evaluate the dose proportionality of LD and CD across the 4 IPX066 dose strengths (23.75–95, 36.25–145, 48.75–195, and 61.25–245 mg of CD-LD). Study 2 was a fixed sequence study evaluating the dose proportionality of LD and CD after administration of 1 and 2 IPX066 61.25- to 245-mg CD-LD capsules. Study 3 was a randomized, crossover study evaluating the effect of a high-fat, high-calorie breakfast and the effect of sprinkling the contents of 2 IPX066 61.25–245 mg LD capsules on applesauce on the pharmacokinetics of LD and CD. The treatment periods in each crossover study were separated by a washout period of at least 6 days.
In study 1 (dose proportionality) and study 2 (1 vs 2 capsules IPX066), all doses were administered under fasting conditions after an overnight fast of at least 10 hours. In study 3, the high-fat, high-calorie breakfast was composed of 2 eggs fried in butter, 2 strips of bacon, 2 slices of toast with butter, 8 oz of hash brown potatoes, and 8 oz of whole milk. During the fed treatment, subjects were required to consume the breakfast within 20 minutes and were administered IPX066 within 30 minutes of starting the breakfast. For the fasted and sprinkled treatments, volunteers fasted overnight for at least 10 hours before dose administration until 4 hours after dosing. Lunch was served 4 hours after drug administration.
Assessments
In each of the 3 studies, the same general study assessments were performed. At screening, a physical examination, urine drug screen, standard laboratory tests (fasting blood chemistry, complete blood count, and urinalysis), pregnancy test, and 12-lead electrocardiogram (ECG) were performed. A medical history was also taken. At the beginning of each treatment period (day 0), a urine pregnancy test, an alcohol test, and a urine drug screen were performed, and a blood sample was obtained for hemoglobin measurements. Resting supine and standing vital signs (heart rate, blood pressure, and respiratory rate) were measured predose and 1, 2, 4, 8, and 12 hours after dosing. Body temperature was measured at screening and before each dosing period. At study termination, a medical history was obtained, and standard laboratory tests, a 12-lead ECG, and vital sign assessments were also performed.
Sample Preparation and Analytical Methods
In all 3 studies, blood samples (6 mL) were collected from each subject to determine plasma concentrations of LD and CD at 0 (predose), 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 7, 8, 10, and 12 hours after dosing. Plasma samples were placed in prechilled polypropylene tubes containing hydrazine dihydrochloride and sodium metabisulfite and were stored at −70°C until analysis. Plasma samples were analyzed within 52 days of sampling. Long-term stability of frozen plasma samples was demonstrated for at least 2 years.
A validated high-performance liquid chromatography tandem mass spectrometry method was used to measure plasma concentrations of LD and CD. Calibration curves were linear for a range of 10 to 2000 ng/mL for LD and 2 to 400 ng/mL for CD (r ≥ 0.99). The interassay precision, as measured by the coefficient of variation (%CV) for quality control samples, ranged across the studies from 2.3% to 7.3% for LD and 2.0% to 7.0% for CD. The interassay accuracy, measured as the percent difference, ranged across the studies from −2.2% to 1.2% for LD and −1.3% to 0.7% for CD.
Pharmacokinetic Analyses
Pharmacokinetic (PK) parameters for LD and CD were estimated by noncompartmental PK methods (Phoenix WinNonlin, version 6.2). The maximum plasma concentration (Cmax) and time to peak concentration (Tmax) were observed values. Apparent elimination half-life (t1/2) was calculated as −(ln2)/k, where k is the slope of log-linear regression of the terminal phase of the concentration-versus-time curve. The area under the plasma concentration-versus-time profile from hour 0 to the last quantifiable concentration at time t (AUCt) was determined by the linear trapezoidal method. The AUC value extrapolated to infinity (AUCinf) was calculated as AUCinf = AUCt + Ct/k, where Ct was the last measurable concentration.
Statistical Analysis
Descriptive statistics (mean and standard deviation) were determined for all PK parameters for each treatment in a study. Statistical analyses were conducted using SAS, version 9.1.3 (Cary, NC). Subjects who completed all treatments in a study were included in the statistical analyses.
Dose Proportionality
A power model (Y = α*(dose)β) as described by Gough et al22 using the modification by Smith et al23 was used to assess the dose proportionality of the PK parameters. In the power model, α is the expected value of Y for a reference dose, and β is the proportionality exponent. A mixed effects model, allowing for random between-subject variability in α and β, was implemented to estimate the proportionality constant and its 90% confidence interval (CI). Dose proportionality was declared if the calculated 90% CI lay within the acceptance range [1 + log(ΘL)/log(R), 1 + log(ΘH)/log(R)], where ΘL and ΘH are the lower and upper limits of the CI and R is the ratio between the highest and lowest doses in the study. A similar approach was used for both study 1 and study 2.
Effect of Food
To assess the effect of food (study 3), an analysis of variance was conducted with factors for dosing condition (fed intact capsule, fasted intact capsule, fasted sprinkled), period, sequence, and subject within sequence. The ratios of the geometric mean and the associated 90% CIs for Cmax, AUCt, and AUCinf between the fed state and fasted state (fed/fasted) and between the fasted sprinkled treatment and fasted intact capsule (sprinkled/intact) were estimated. Absence of a food effect was to be concluded if the point estimate and the 90% CI for the ratio of the geometric means for Cmax and for AUCinf were contained within the prespecified acceptance criteria of 80% to 125%. A nonparametric Wilcoxon rank test was performed on the untransformed Tmax values. Assuming a root mean square error of 0.15, a sample size of 18 subjects was estimated to detect a 20% difference in the log-transformed AUC with 90% power so that the ratio of the mean AUC for any 2 treatments fell within an interval of 80% to 125% based on two 1-sided tests with an α = 0.05.
Safety Assessments
Adverse events (AEs) were monitored throughout the study in accordance with International Conference of Harmonization Guidance.24 Subjects were asked a nonspecific question regarding how they were feeling periodically during the study. Adverse events were assessed in terms of severity (mild, moderate, severe) and relationship to study drug by the investigators (James Freeman, MD, and Ramón Vargas, MD, MPH). Additional safety assessments included routine physical examination, vital signs (blood pressure and pulse), ECG, and routine laboratory tests.
RESULTS
Subject Baseline Characteristics
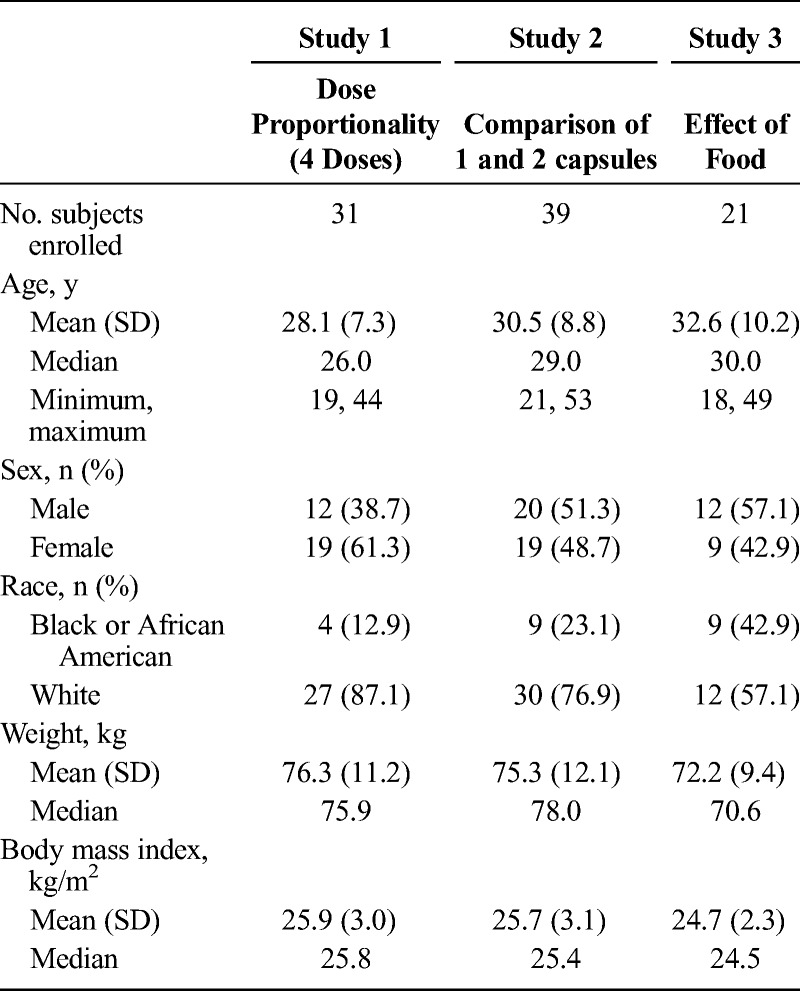
A total of 31 subjects were enrolled, and 28 subjects completed the dose proportionality study (study 1). Thirty-nine subjects were enrolled, and 34 subjects completed study 2. Twenty-one subjects were enrolled, and 19 subjects completed all treatments in study 3. Baseline demographics for all studies are presented in Table 2.
TABLE 2.
Baseline Characteristics of Subjects Across Studies
Pharmacokinetics
Dose Proportionality
Mean plasma concentration-time curves after single doses of each of the 4 strengths of IPX066 capsules (95- to 245-mg LD; study 1) showed a dose-dependent increase in LD and CD concentrations (Fig. 1). The LD plasma concentration-time profiles were similar across the dose strengths; all had a rapid increase with initial peak concentrations noted within 1 hour of dosing followed by concentrations that were maintained for approximately 4 to 5 hours. Table 3 summarizes the LD and CD PK parameters after IPX066 dosing. Peak concentrations and AUC values of LD and CD increased in a dose-proportional manner. Mean half-life values were similar across the doses evaluated. Median Tmax values ranged from 2.8 to 4 hours for LD, consistent with a flat plasma profile.
FIGURE 1.
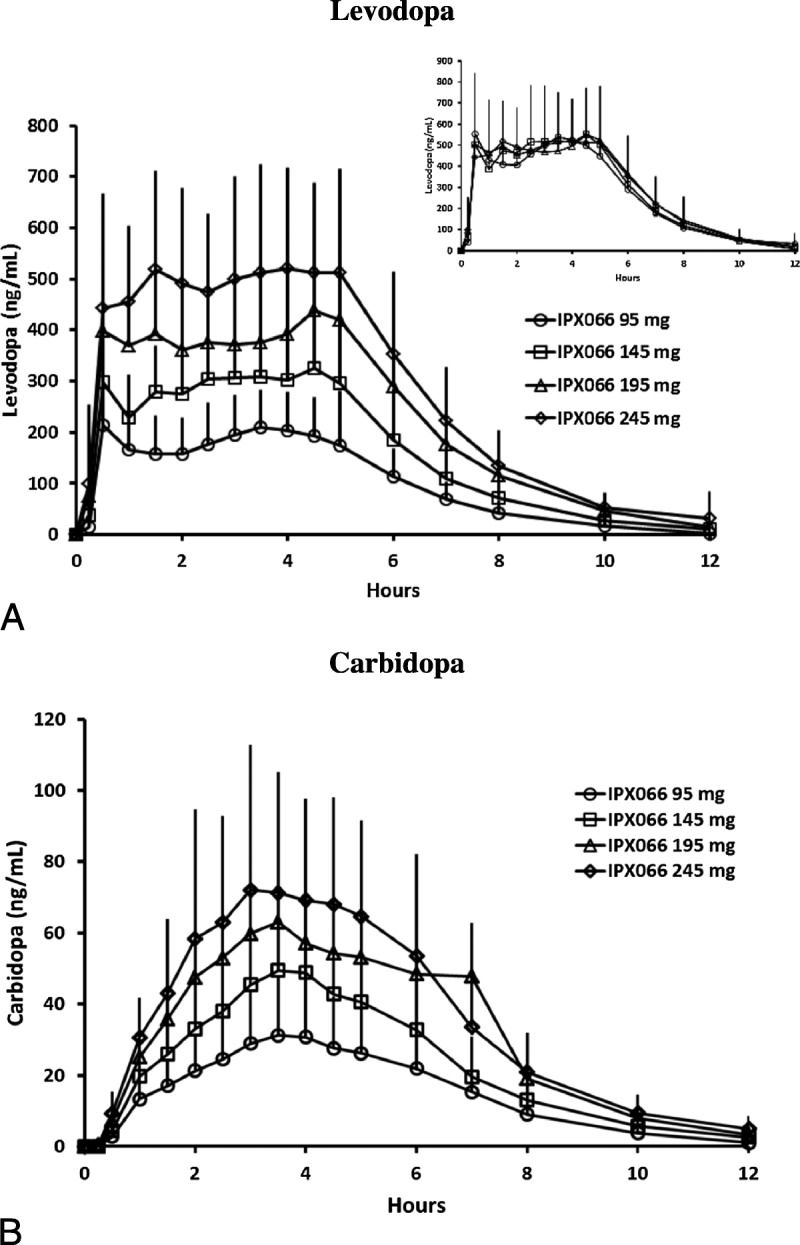
Mean (standard deviation) plasma concentration-time profiles for LD (A) and CD (B) after oral administration of each of the 4 strengths of IPX066 capsules (95-245 mg of LD)—study 1. The inset shows LD concentrations after dose normalization to 245-mg LD.
TABLE 3.
Single-Dose PK Parameters and Dose Proportionality of LD and CD After Oral Administration of IPX066 Capsules in Healthy Subjects (N = 28): Study 1
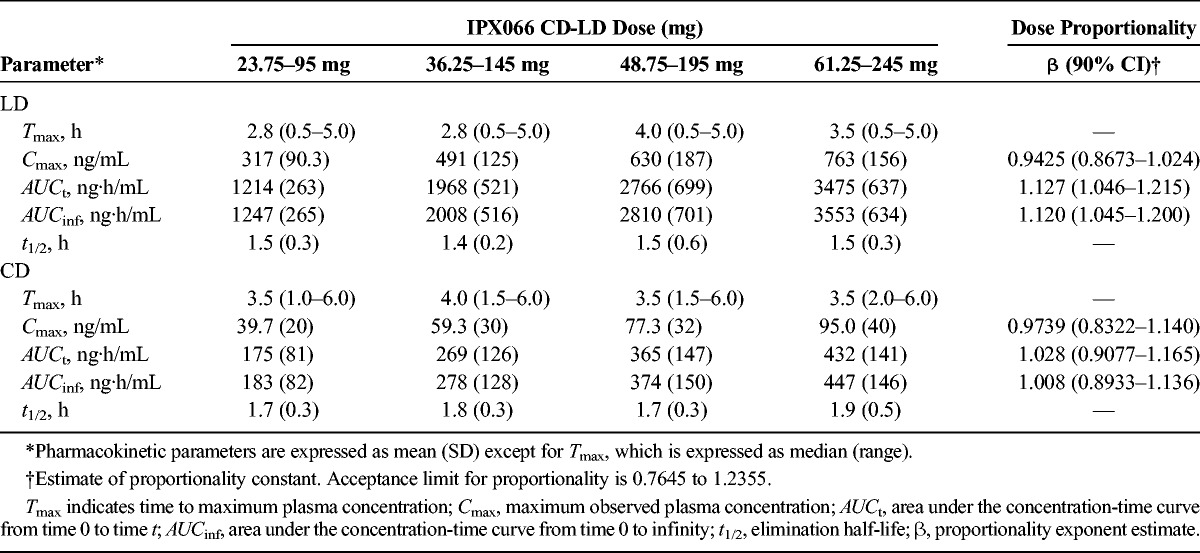
The proportionality coefficient for Cmax, AUCt, and AUCinf for LD were 0.943 (90% CI, 0.867–1.02), 1.13 (90% CI, 1.05–1.22), and 1.12 (90% CI, 1.05–1.20), respectively (Table 3). The power model analysis indicated that the lower and upper limits of the 90% CI for the proportionality coefficient (β) were within acceptance intervals (0.7645–1.2355) for both LD and CD.
The LD and CD pharmacokinetics after a single 245-mg IPX066 capsule (245-mg LD) and two 245-mg capsules (total dose, 490-mg LD) are summarized in Table 4. The LD plasma-concentration profile was similar to that seen in the dose-proportionality study. Analysis of dose proportionality using the power model indicated that the lower and upper limits of the 90% CI for the proportionality coefficient (β) were within acceptance intervals (0.6781–1.3219) for Cmax, AUCt, and AUCinf for CD and for Cmax and AUCt for LD. The 90% CI for LD AUCinf was slightly higher than the upper limit of the acceptance range.
TABLE 4.
PK Parameters and Dose Proportionality of LD and CD After Single Oral Doses of 1 and 2 Capsules of IPX066 (245-mg LD) in Healthy Subjects (N = 34): Study 2
Effect of Food
After oral administration of two 245-mg IPX066 capsules (490-mg LD) in the fasted state, LD concentrations increased rapidly reaching Tmax at a median of 1.5 hours (Table 5). Dosing after a high-fat, high-calorie breakfast resulted in a slower absorption of LD (Fig. 2), and the median Tmax occurred at 7 hours (Table 5). A nonparametric assessment of the Tmax value suggested that the LD time to peak was delayed when IPX066 was administered with a high-fat, high-calorie breakfast. The geometric mean ratio (fed vs fasted) for Cmax (90% CI) for LD was 79.41 (71.90–87.70), and for AUCinf, the ratio was 112.75 (104.74–121.37).
TABLE 5.
Effect of Food and Sprinkling the Capsule Contents on Applesauce on the PKs of LD and CD After Single Oral Administration of 2 IPX066 245-mg Capsules in Healthy Subjects (N = 19): Study 3
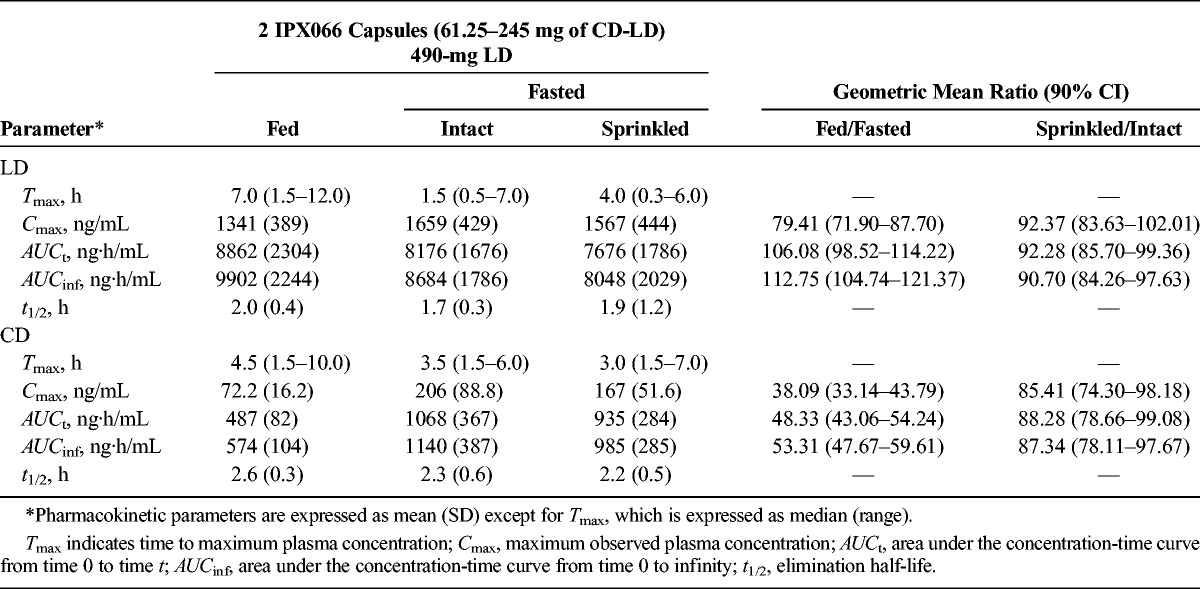
FIGURE 2.
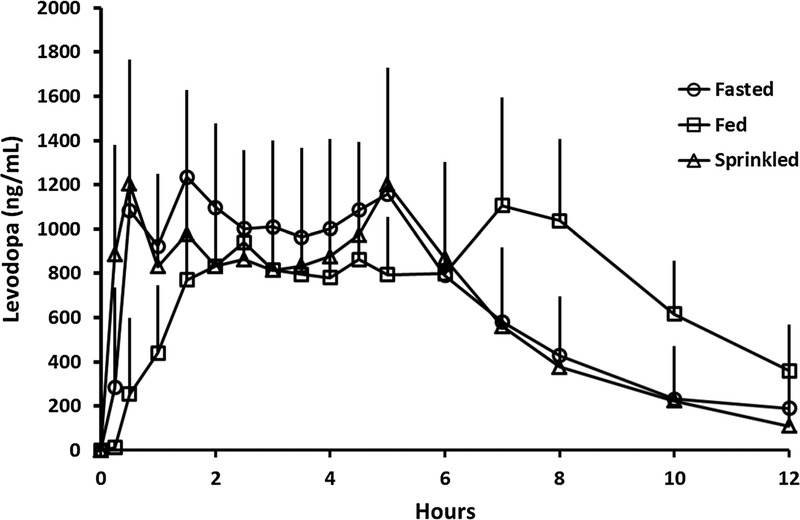
Mean (standard deviation) plasma concentration-time profiles for LD after single oral administration of 2 IPX066 245-mg capsules in healthy subjects under fasted (intact and sprinkled on applesauce) and fed conditions—study 3.
When the contents of two 245-mg LD IPX066 capsules (total LD dose, 490 mg) were sprinkled on applesauce and taken in the fasted state, the LD plasma concentration profile (Fig. 2) and LD PK parameters (Table 5) were comparable with those when IPX066 capsules were taken intact in the fasted state. The ratio of the geometric means (sprinkled/intact) for LD Cmax and for AUCinf was 92.37% and 90.70%, respectively, and the 90% CIs were well within the range of 80% to 125%. A nonparametric assessment suggested that there was no difference in the Tmax values when the IPX066 capsule contents were sprinkled on applesauce compared with when IPX066 capsules were taken intact.
Safety and Tolerability
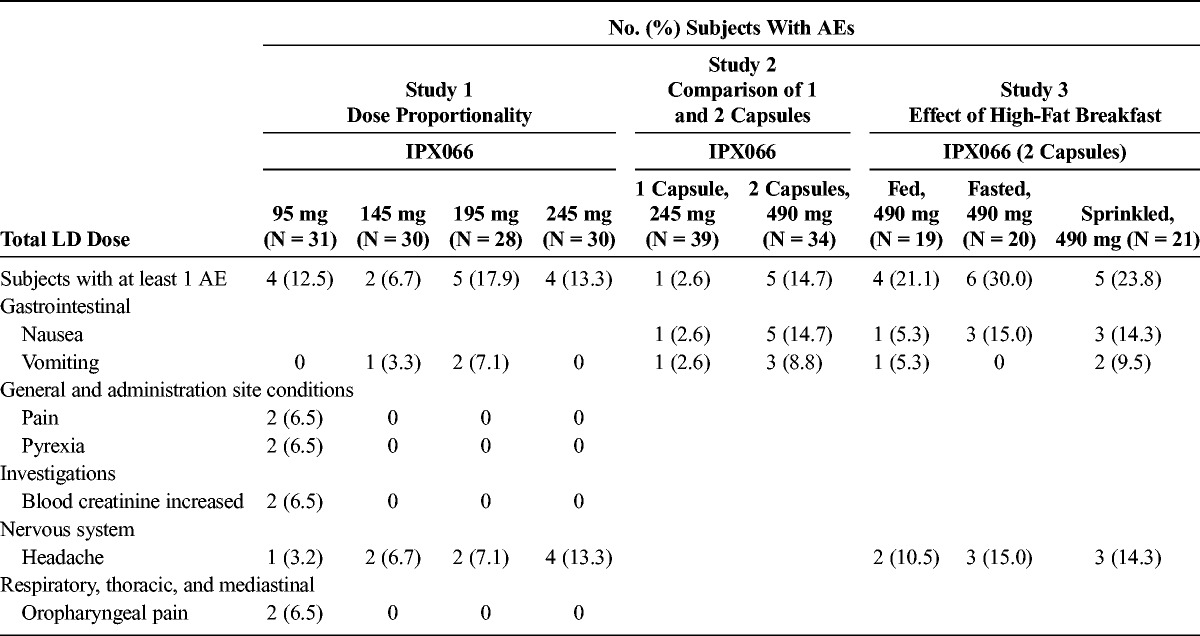
All subjects who received at least 1 treatment were included in the safety evaluation. No serious AEs were reported. Table 6 summarizes the AEs reported in 2 or more subjects during any treatment for the 3 studies. In study 1, AEs reported by 2 or more subjects included vomiting, pain, pyrexia, increase in blood creatinine, headache, and oropharyngeal pain. All AEs, except 1 report of moderate vomiting (IPX066 195 mg, possibly related), were categorized as mild in severity. In study 2, AEs reported in 2 or more subjects included nausea and vomiting. More subjects reported AEs when they received 2 capsules compared with 1 capsule. All AEs were categorized as mild, except 2 reports of nausea (both after 2 capsules and possibly related) and 1 report of vomiting (2 capsules, not related), which were categorized as moderate. In study 3, AEs reported by 2 or more subjects included nausea, vomiting, and headache. All AEs were categorized as mild in severity, except 1 report each of vomiting during the fed and sprinkled treatment and 1 report of nausea during the fasted treatment, which were all categorized as moderate in severity and treatment related. Overall, IPX066 was generally well tolerated in all 3 studies in this population of healthy volunteers.
TABLE 6.
AEs Reported in 2 or More Subjects in Any Group
DISCUSSION
IPX066 capsules are available in 4 dose strengths: 23.75–95, 36.25–145, 48.75–195, and 61.25–245 mg of CD-LD. The PK studies presented here were designed to describe the dose proportionality of IPX066; to characterize the effect of a high-fat, high-calorie meal on the pharmacokinetics of IPX066; and to study the effect of sprinkling the capsule contents on applesauce.
After oral administration of IPX066 capsules, the absorption of LD was fast, reaching initial peak concentrations by approximately 1 hour after which LD plasma concentrations were sustained for approximately 4 to 5 hours before they began to decline. Carbidopa plasma concentrations increased slowly, reaching Cmax at approximately 3.5 to 4 hours, and declined subsequently.
Levodopa and CD AUC and Cmax showed dose-proportional pharmacokinetics for Cmax, AUCt, and AUCinf over the range of dose strengths evaluated (95-245 mg of LD). Levodopa plasma concentrations were superimposable after dose normalization indicating consistent, predictable pharmacokinetics across the dose strengths (Fig. 1, inset). Two IXP066 245-mg LD capsules showed dose-proportional pharmacokinetics compared with a single capsule for Cmax and for AUCt. Yeh and colleagues25 have also shown that, for CD-LD controlled release (Sinemet CR), LD peak concentrations and absorption are dose proportional between 1 and 2 tablets.
Food has been shown to alter gastric pH, gastric emptying, and gastrointestinal motility.26 Reports on the effect of food on LD absorption are mixed. In a study in healthy volunteers with controlled-release CD-LD (Sinemet CR), Yeh and colleagues25 (1989) reported that food increased the bioavailability of LD by approximately 1.48-fold and decreased the bioavailability and peak concentration of CD. A high-fat breakfast decreased the Cmax, delayed Tmax, and had no effect on the AUC for a dual-release formulation of LD (Madopar DR).19 In a study evaluating a hydrodynamically balanced formulation of LD (Madopar HBS), Malcolm et al17 reported that the AUC was similar after oral dosing with a standard meal and in the fasted state. In contrast, in another study evaluating the influence of a standard meal in patients with Parkinson disease, Roos and colleagues27 found that the mean LD concentrations were significantly higher in the fasted condition compared with the nonfasted condition. Peak LD concentrations were higher, and Tmax tended to occur somewhat earlier in the fasted state. In a study in patients with Parkinson disease, administration of controlled-release CD-LD 30 minutes after a standardized low-protein meal resulted in a significant delay in LD absorption while Cmax was unaffected.28 The delay in absorption also resulted in a latency in tapping rate.
In the current study, we noted that administration of IPX066 with a high-fat, high-calorie breakfast led to an approximate 2-hour delay in LD absorption and a small (∼13%) increase in LD AUC. Peak concentration was reduced approximately 21% compared with the fasted state. Similar to observations made by Yeh et al25 that food decreased the absorption of CD with Sinemet CR, this study also found that a high-fat breakfast decreased absorption of CD but this did not adversely affect LD bioavailability from IPX066. Initial absorption of LD was delayed in the presence of a high-fat meal, and the median Tmax occurred later than in the fasting state. Care must be taken in interpreting Tmax values from concentration profiles with multiple peaks or extended-release formulation containing multiple components that are designed to achieve a wide, almost flat peak.29
IPX066 is a multiparticulate capsule formulation with a distinct combination of different types of beads, designed to provide a characteristic LD plasma profile. As a result, unlike some other formulations, IPX066 should not be chewed, divided, or split. The availability of several dose strengths provides adequate dosing flexibility. The results from this study demonstrate that the IPX066 capsule contents may be sprinkled on applesauce without affecting LD pharmacokinetics compared with the intact capsule. This may facilitate dosing for patients who have trouble swallowing intact tablets or capsules. There was a reduction in CD Cmax and AUC upon sprinkling compared with the intact capsule. However, the decrease in CD absorption did not adversely affect the pharmacokinetics of LD.
CONCLUSIONS
IPX066 exhibits dose-proportional pharmacokinetics over the dose range of 95- to 490-mg LD. A high-fat, high-calorie breakfast does not affect the systemic exposure to LD but may delay the onset of LD plasma concentrations. Sprinkling the IPX066 capsule contents onto soft foods, such as applesauce, does not affect the pharmacokinetics of LD.
Footnotes
Conflicts of Interest and Source of Funding: The studies were funded by Impax Laboratories, Inc. At the time of their contribution, all authors were employees of Impax Laboratories, Inc, and held Impax stock.
REFERENCES
- 1. LeWitt PA. Levodopa for the treatment of Parkinson's disease. N Engl J Med. 2008; 359: 2468– 2476. [DOI] [PubMed] [Google Scholar]
- 2. Schapira AH, Emre M, Jenner P, et al. Levodopa in the treatment of Parkinson's disease. Eur J Neurol. 2009; 16: 982– 989. [DOI] [PubMed] [Google Scholar]
- 3. Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord. 2015; 30: 80– 89. [DOI] [PubMed] [Google Scholar]
- 4. Hauser RA, McDermott MP, Messing S. Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch Neurol. 2006; 63: 1756– 1760. [DOI] [PubMed] [Google Scholar]
- 5. Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol. 2006; 5: 677– 687. [DOI] [PubMed] [Google Scholar]
- 6. Hardie RJ, Malcolm SL, Lees AJ, et al. The pharmacokinetics of intravenous and oral levodopa in patients with Parkinson's disease who exhibit on-off fluctuations. Br J Clin Pharmacol. 1986; 22: 429– 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Syed N, Murphy J, Zimmerman T, Jr, et al. Ten years' experience with enteral levodopa infusions for motor fluctuations in Parkinson's disease. Mov Disord. 1998; 13: 336– 338. [DOI] [PubMed] [Google Scholar]
- 8. Nyholm D, Aquilonius SM. Levodopa infusion therapy in Parkinson disease: state of the art in 2004. Clin Neuropharmacol. 2004; 27: 245– 256. [DOI] [PubMed] [Google Scholar]
- 9. Poewe W, Antonini A. Novel formulations and modes of delivery of levodopa. Mov Disord. 2015; 30: 114– 120. [DOI] [PubMed] [Google Scholar]
- 10. LeWitt PA, Nyholm D. New developments in levodopa therapy. Neurology. 2004; 62( suppl 1): S9– S16. [DOI] [PubMed] [Google Scholar]
- 11. LeWitt PA, Jennings D, Lyons KE, et al. Pharmacokinetic-pharmacodynamic crossover comparison of two levodopa extension strategies. Mov Disord. 2009; 24: 1319– 1324. [DOI] [PubMed] [Google Scholar]
- 12. Stocchi F, Quinn NP, Barbato L, et al. Comparison between a fast and a slow release preparation of levodopa and a combination of the two: a clinical and pharmacokinetic study. Clin Neuropharmacol. 1994; 17: 38– 44. [DOI] [PubMed] [Google Scholar]
- 13. Pahwa R, Lyons K, McGuire D, et al. Early morning akinesia in Parkinson's disease: effect of standard carbidopa/levodopa and sustained-release carbidopa/levodopa. Neurology. 1996; 46: 1059– 1062. [DOI] [PubMed] [Google Scholar]
- 14. Stocchi F, Rascol O, Kieburtz K, et al. Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol. 2010; 68: 18– 27. [DOI] [PubMed] [Google Scholar]
- 15. Pahwa R, Lyons KE, Hauser RA, et al. Randomized trial of IPX066, carbidopa/levodopa extended release, in early Parkinson's disease. Parkinsonism Relat Disord. 2014; 20: 142– 148. [DOI] [PubMed] [Google Scholar]
- 16. Hauser RA, Hsu A, Kell S, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol. 2013; 12: 346– 356. [DOI] [PubMed] [Google Scholar]
- 17. Malcolm SL, Allen JG, Bird H, et al. Single-dose pharmacokinetics of Madopar HBS in patients and effect of food and antacid on the absorption of Madopar HBS in volunteers. Eur Neurol. 1987; 27( suppl 1): 28– 35. [DOI] [PubMed] [Google Scholar]
- 18. Baruzzi A, Contin M, Riva R, et al. Influence of meal ingestion time on pharmacokinetics of orally administered levodopa in parkinsonian patients. Clin Neuropharmacol. 1987; 6: 527– 537. [DOI] [PubMed] [Google Scholar]
- 19. Crevoisier C, Zerr P, Calvi-Gries F, et al. Effects of food on the pharmacokinetics of levodopa in a dual-release formulation. Eur J Pharm Biopharm. 2003; 55: 71– 76. [DOI] [PubMed] [Google Scholar]
- 20. Robertson DR, Renwick AG, Wood ND, et al. The influence of levodopa on gastric emptying in man. Br J Clin Pharmacol. 1990; 29: 47– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Müller T, Erdmann C, Bremen D, et al. Impact of gastric emptying on levodopa pharmacokinetics in Parkinson disease patients. Clin Neuropharmacol. 2006; 29: 61– 67. [DOI] [PubMed] [Google Scholar]
- 22. Gough K, Hutchinson M, Keene O, et al. Assessment of dose proportionality: report from statisticians in the pharmaceutical industry/pharmacokinetics UK joint working party. Drug Info J. 1995; 29: 1039– 1048. [Google Scholar]
- 23. Smith BP, Vandenhende FR, DeSante KA, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000; 17: 1278– 1283. [DOI] [PubMed] [Google Scholar]
- 24. ICH tripartite guideline E2A Clinical safety data management: definitions and standards for expedited reporting. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf. Accessed September 3, 2015.
- 25. Yeh KC, August TF, Bush DF, et al. Pharmacokinetics and bioavailability of Sinemet CR: a summary of human studies. Neurology. 1989; 39( suppl 2): 25– 38. [PubMed] [Google Scholar]
- 26. Fleisher D, Li C, Zhou Y, et al. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin Pharmacokinet. 1999; 36: 233– 254. [DOI] [PubMed] [Google Scholar]
- 27. Roos RA, Tijssen MA, van der Velde EA, et al. The influence of a standard meal on Sinemet CR absorption in patients with Parkinson's disease. Clin Neurol Neurosurg. 1993; 95: 215– 219. [DOI] [PubMed] [Google Scholar]
- 28. Contin M, Riva R, Martinelli P, et al. Effect of meal timing on the kinetic-dynamic profile of levodopa/carbidopa controlled release [corrected] in parkinsonian patients. Eur J Clin Pharmacol. 1998; 54: 303– 308. [DOI] [PubMed] [Google Scholar]
- 29. Endrenyi L, Tothfalusi L. Metrics for the evaluation of bioequivalence of modified-release formulations. AAPS J. 2012; 14: 813– 819. [DOI] [PMC free article] [PubMed] [Google Scholar]


