Figure 4.
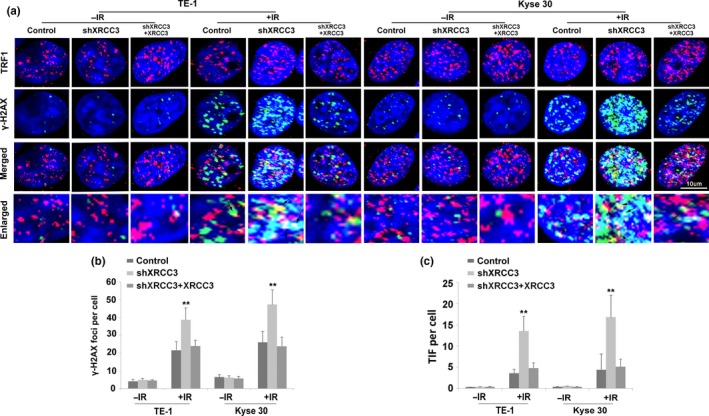
Silencing of XRCC3 promotes ionizing radiation (IR)‐induced DNA damage and telomere dysfunction in both Kyse30 and TE‐1 cells. (a) Silencing of XRCC3 by shXRCC3 increased the percentage of telomere dysfunction induced foci (TIF)‐positive cells among both TE‐1 and Kyse30 cells. Cells were fixed and double stained with an anti‐TRF1 mouse monoclonal antibody to mark telomeres (red) and an anti‐phosphorylated histone H2A.X (Ser‐139) rabbit monoclonal antibody to indicate DNA damage‐activated γH2AX foci (green). Nuclei were counterstained with DAPI (blue). Merged images show DNA damage at telomeres. Arrows indicate double‐stained areas (yellow). (b) Quantification of the average numbers of IR‐induced γH2AX foci per cell. (c) Quantification of the average numbers of IR‐induced TIF per cell. After replenishment of XRCC3 in both XRCC3‐silenced KYSE30 and TE‐1 cells, the altered levels of IR‐induced DNA damage and telomere dysfunction were recovered. 48 h after irradiation, cells were fixed to perform an immunofluorescence assay. All data are derived from three individual experiments. Data represent mean values and SE. (Bar equals 10 um. *P < 0.05; **P < 0.01, Student's t‐test.)
