Figure 1.
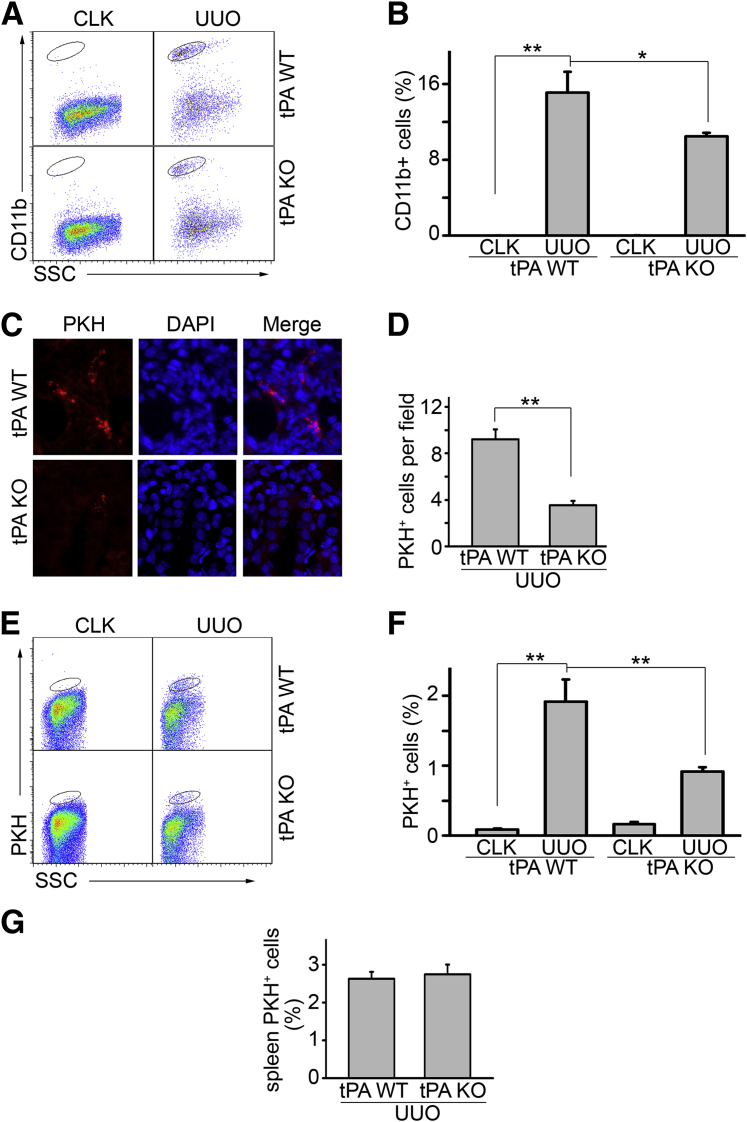
tPA promotes macrophage infiltration and migration in vivo. Unilateral ureteral obstruction (UUO) was performed in tPA wild-type (WT) and knockout (KO) mice (n = 4 mice per group) for 7 days. Single-cell suspensions were prepared from whole kidneys and were subjected to flow cytometry analysis using anti-CD11b antibody to identify macrophages. A: Representative flow cytometry analysis. Cells in the ovals were CD11-positive macrophages. Contralateral unobstructed kidneys served as controls. B: Quantitation of the ratio of CD11b macrophages in the gated cells. C: Fluorescence microscopy of PKH26-labeled macrophages in the fibrotic kidneys. Bone marrow–derived macrophages from tPA KO mice were labeled by PKH26 and intravenously transferred into tPA KO and WT mice, followed by UUO for 7 days. PKH-labeled macrophages in the obstructed kidneys were counterstained by DAPI and quantitated under the fluorescence microscope. D: Quantification of PKH26-positive macrophages. Units were expressed as number of PKH26-positive macrophages per ×400 field, 5 fields per mouse. E: Flow cytometry analysis of the PKH26-positive macrophages. Some WT and tPA KO mice receiving adoptive PKH26-positive macrophage transfer were subjected to flow cytometry analysis. Cells in the ovals are PKH26-labeled macrophages. Data are representative from one of the three animals analyzed. Contralateral unobstructed kidneys served as controls. F: Quantitation of flow cytometry analysis. G: Flow cytometry analysis of the ratio of PKH26-positive CD11b macrophages in the gated cells of the spleens from tPA KO and WT mice with UUO for 7 days. No statistical significance exists. ∗P < 0.05, ∗∗P < 0.01. n = 3 (F and G, mice per group); n = 4 (B, mice per group); n = 5 (C and D, mice per group). Original magnification, ×400 (C). CLK, contralateral unobstructed kidneys; SSC, side-scattered light.