Abstract
Circulating tumor cells and disseminated tumor cells (DTCs) are of great interest because they provide a minimally invasive window for assessing aspects of cancer biology, including tumor heterogeneity, a means to discover biomarkers of disease behavior, and a way to identify and prioritize therapeutic targets in the emerging era of precision oncology. However, the rarity of circulating tumor cells and DTCs poses a substantial challenge to the consistent success in analyzing their molecular features, including genomic aberrations. Herein, we describe optimized and robust methods to reproducibly detect genomic copy number alterations in samples of 2 to 40 cells after whole-genome amplification with the use of a high-resolution single-nuclear polymorphism-array platform and refined computational algorithms. We have determined the limit of detection for heterogeneity within a sample as 50% and also demonstrated success in analyzing single cells. We validated the genes in genomic regions that are frequently amplified or deleted by real-time quantitative PCR and nCounter copy number quantification. We further applied these methods to DTCs isolated from individuals with advanced prostate cancer to confirm their highly aberrant nature. We compared copy number alterations of DTCs with matched metastatic tumors isolated from the same individual to gain biological insight. These developments provide high-resolution genomic profiling of single and rare cell populations and should be applicable to a wide-range of sample sources.
Tumor heterogeneity complicates our understanding of the biological mechanism of cancer and presents challenges for effective diagnostic and therapeutic approaches in the clinic. Among techniques that help define the alterations present in all tumor subpopulations, methods to detect and isolate tumor cells from the blood or bone marrow of cancer patients provide new and relatively noninvasive alternatives for sampling solid tumors and are referred to as liquid biopsies.1 Circulating tumor cells (CTCs) are shed from tumors into the blood and provide an index of the current tumor burden.2 Although the half-life of a CTC is short (approximately 2 hours),3 disseminated tumor cells (DTCs) have effectively migrated to the bone marrow, where they may remain dormant for up to several decades but may gain metastatic potential eventually.4 Quantification of both CTCs and DTCs has shown promise as novel diagnostics to measure tumor burden and to assess the risk of relapse after initial therapies in breast and prostate cancers.5, 6, 7 A recent meta-analysis of 33 clinical studies supported the prognostic value of CTCs/DTCs for survival outcome in prostate cancer.8 Molecular characterization of CTCs and DTCs will provide information about genomic aberrations, expression profiles, or other cellular perturbations that may better predict treatment response or disease outcome.
The rarity of CTC/DTCs poses a substantial challenge to the consistent success in analyzing the genome of these cells. Previous studies were limited to low-resolution analyses of a subset of genes or genomic loci.9 Recent advances in the genome-wide analysis of single cells with next-generation sequencing approaches have offered great promise in the clinical application of CTC/DTCs as prognostic and predictive biomarkers, especially focused on somatic mutations.10, 11 Nevertheless, the few whole-genome profiling studies that examine somatic copy number aberrations (SCNAs) published thus far used relatively low-resolution bacterial artificial chromosome-based or oligonucleotide-based comparative genome hybridization techniques.12, 13, 14, 15 A robust, reproducible, cost-effective, and high-resolution genomic profiling method is sorely desired by many researchers.
Here, we describe in detail an optimized method for consistent and robust genome-wide profiling of prostate cancer DTCs that yields high resolution for SCNAs on samples of 2 to 40 cells from individual patients. With the use of well-characterized cell lines, we have optimized methods for the whole-genome amplification (WGA) and single-nuclear polymorphism (SNP) array analysis of archived samples. We refined computational methods to reduce noise and to improve the segmentation and copy number (CN)-calling methods for data generated from WGA samples. We then applied these methods to DTCs isolated from patients with advanced prostate cancer to confirm the highly aberrant nature of these cells and to compare SCNAs with those in matched metastatic tumors from the same individual. We validated genes in genomic regions that are frequently amplified or deleted with real-time quantitative PCR and nCounter CN quantification. These developments provide high-resolution genomic profiling of single and rare cell populations and should be applicable to a wide-range of sample sources, including CTCs, formalin-fixed, paraffin-embedded-derived cells, and embryonic cell testing.
Materials and Methods
Cell Lines and Patient Samples
The LNCaP prostate adenocarcinoma cell line was maintained according to ATCC (Manassas, VA) instructions. A male velocardiofacial (VCF) syndrome cell line GM07939B [46,XY,del(22)(q11.21q11.22)] was obtained from the Coriell Institute (Camden, NJ) and cultured according to the provided protocols. Ten normal female lymphoblast reference (NFR) lines were a gift from Dr. Barbara Trask (Lawrence Livermore National Laboratory, Livermore, CA) and were previously described.16 All samples that contained ≤40 cells were collected with a micromanipulator as previously described.12 Bulk genomic DNA from cell lines was extracted from cell pellet that contained approximately 2 × 106 cells, using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA).
Metastatic tumor samples from prostate cancer patients were collected at the University of Washington from autopsies performed within 6 hours of death under the aegis of the rapid autopsy program.17 All tissues were frozen immediately and stored at −80°C. Hematoxylin and eosin-stained tissue sections were reviewed by a pathologist for verification of histology. This study was approved by the institutional review board at the Fred Hutchinson Cancer Research Center and University of Washington. The data discussed in this publication were deposited in National Center for Biotechnology Information's Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo; accession number GSE73180).
Isolation of DTCs
The methods to isolate DTCs from bone marrow aspirates were described previously.6, 18 Briefly, 10 mL of bone marrow was aspirated from the right iliac crest and combined with 10 mL of 6% sodium citrate. Samples were processed as soon as possible. Aspirates were placed over 15 mL of Ficoll-Isopaque 1.077 g/mL (Accurate Chemical, Westbury, NY) and centrifuged to yield a mononuclear cell layer. Two rounds of immunomagnetic selection (Miltenyi Biotec, San Diego, CA) were used to enrich for DTCs. First, negative selection of leukocytes, megakaryocytes, and platelets was performed with antibodies to CD45 and CD61. Second, immunomagnetic beads coated with an anti–epithelial cell adhesion molecule (EpCAM) antibody (clone HEA-125/CD326) were used to positively select cells that expressed epithelial antigens. The eluted fraction contained a cell mixture enriched for cells of epithelial origin.
To identify and isolate DTCs, the enriched cell population was immunostained with fluorescein isothiocyanate-labeled BerEP4 clone of the anti-EpCAM antibodies (Dako, Carpinteria, CA), which bind to a different epitope on human EpCAM than the antibody used for positive selection.6 A phycoerythrin-conjugated anti-CD45 antibody was also used to counterstain leukocytes. Cells were viewed under fluorescent light with the use of an inverted microscope. DTCs were defined as CD45− cells that have intermediate-to-high labeling with anti-EpCAM. EpCAM+/CD45− cells were kept on ice and viewed under fluorescent light, and intact cells with a clearly defined EpCAM-labeled membrane were isolated with a micromanipulator and were dispensed into individual snap-top PCR tubes that contained 10 μL of water. Samples were frozen immediately on dry ice and stored at −80°C until WGA. We were able to generate data of comparable quality from samples stored up to 12 years relative to DTCs acquired within the past 2 years.
DNA Extraction from Bone Metastases and Laser-Captured Materials
Bone, lymph node, and liver metastases were laser capture microdissected with an Arcturus Veritas instrument and collected on CapSure Macro LCM Caps (Life Technologies, Carlsbad, CA). DNA was isolated with QiaAMP DNA Blood Micro kit (Qiagen) according to manufacturer's protocol with the following modifications: samples were incubated with 15 μL buffer ATL and 10 μL proteinase K (Life Technologies, Carlsbad, CA) for 14 to 20 hours at 37°C with circular rotation. Lysates were collected to a 1.5-mL microfuge tube, and a second round of extraction was repeated for 4 to 6 hours. Lysates were combined with 50 μL of buffer AL, and DNA was extracted according to protocol. DNA was eluted in a total of 50 μL of a 1 AE: 4 water solution in two sequential extractions that contained 30 μL, followed by 20 μL. DNA was quantified with Quant-iT PicoGreen dsDNA assay (Invitrogen) on a Fluroskan Ascent (ThermoScientific, Waltham, MA).
Optimized WGA Procedure
For WGA of samples that contained ≤40 cells, PCR tubes with cells were thawed in a 96-well rack prechilled on ice, and contents were briefly collected by centrifuging at 16,000 × g for 1 minute. Tubes were dried completely in a SpeedVac on low or medium setting, with monitoring to avoid over-drying samples. To control for possible contamination throughout the WGA process, a water-only sample was also dried in the SpeedVac and was treated in the same manner as tubes that contained cell aliquots. The WGAs were performed with the PicoPLEX WGA kit (Rubicon Genomics, Ann Arbor, MI) according to the instructions. All steps that involved WGA were performed in a manner to minimize possible contamination and to maintain good PCR practices. When possible, individual samples and reagents were handled by metal tweezers, opened only in a PCR workstation (AirClean Systems, Creedmoor, NC), and samples were kept on ice whenever possible.
Briefly, DTC samples that were originally collected in a 0.5-mL microfuge tube in 5 to 10 μL H2O. After sample drying in a SpeedVac, cell samples were resuspended in 5 μL of cell extraction buffer. Then, 5 μL of freshly prepared extraction cocktail was added to each tube. Samples were mixed on a vortex, centrifuged, and incubated in a PTC-100 thermocycler (MJ Research Inc., St. Bruno, QC, Canada) at 75°C for 10 minutes and then at 95°C for 4 minutes. Samples were collected by centrifugation, had 5 μL of freshly prepared Pre-Amp Cocktail added, and were mixed on a vortex and centrifuged. Samples were incubated in a thermocycler as follows: one cycle at 95°C for 2 minutes, followed by 12 cycles of 95°C for 15 seconds, 15°C for 50 seconds, 25°C for 40 seconds, 35°C for 30 seconds, 65°C for 40 seconds, and 75°C for 40 seconds. Samples were then placed immediately on ice and collected by centrifugation, and 60 μL of freshly prepared amplification cocktail were added. Samples were mixed well on a vortex, briefly centrifuged, then amplified in a thermocycler as follows: 95°C for 2 minutes, followed by 14 cycles of 95°C for 15 seconds, 65°C for 1 minute, and 75°C for 1 minute.
All genomic DNA from laser-captured tissue was amplified with the WGA2 kit (GenomePlex Complete Whole Genome Amplification Kit; Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. If original DNA samples were <1.0 ng/μL starting concentration, they were dried in a SpeedVac and resuspended in sterile water to a concentration of 1.0 ng/μL.
After WGA, samples were purified with Qiagen PCR purification columns according to the manufacturer's directions, with the following modifications: after initial wash with 0.75 mL Buffer PE, tubes were transferred to an empty 1.5-mL microfuge tube and completely dried by centrifuging at 16,000 × g for 3 minutes; DNA was eluted in a room temperature 1 EB:1 water mixture by adding 30 μL to column, allowed to stand at room temperature for up to 3 minutes, then centrifuged at 16,000 × g for 1 minute, followed by a second elution with 25 μL, for a total of 55 μL per sample.
Prearray Quality Control Analysis
After WGA, 250 ng of DNA were assessed on a 2% agarose gel. Samples that showed amplification of predominantly smaller fragments (<500 bp) were considered to have failed WGA. In addition, a multiplex PCR reaction to coamplify 100-, 200-, 300-, and 400-bp fragments from unique regions of the GAPDH locus was established (primer sequences are listed in T) to examine whether different sizes of the DNA fragments were properly amplified. PCR reactions that contained 80 ng WGA DNA in 25-μL total reaction volume were amplified with FastStart Taq Polymerase Kit (Roche, Indianapolis, IN).
Optimization of SNP Array and Post SNP Array Quality Control Measures
Several SNP arrays were compared for performance on WGA DNA samples, including CytoSNP-12 and Omni1-Quad bead chips (Illumina, San Diego, CA) and NspI 250k, SNP6.0, and CytoScanHD arrays (Affymetrix, Santa Clara, CA). Overall, for DTCs, Picoplex-amplified samples of ≤40 cells performed best on the CytoSNP-12 bead chip; higher probe density arrays yielded data with more noise and interprobe variability (data not shown). For WGA2-amplified samples, Omni1-Quad bead chips were selected for best performance and low interprobe variability.
Human CytoSNP-12 bead arrays were processed according to the manufacturer's directions at the Fred Hutchinson Cancer Research Center Genomics Resource. Genotyping calls were generated with the GenomeStudio Software version 2011.1 (Illumina) with the Human CytoSNP-12_v2 Hap Map genotype cluster definitions. Analysis of WGA DNA samples in GenomeStudio Software used the WGA sample set for clustering. Unamplified controls were analyzed with Illumina's catalog cluster results. Normalized logR values for each probe location were exported for analysis with Nexus Copy Number 6.0 software (Biodiscovery, El Segundo, CA) or Fused Lasso.
Optimization of Bioinformatics Tools
With the use of the NFR set and LNCaP cell aliquots, we established variables for smoothing interprobe variation and segmentation of probes into regions with similar values of average log2 ratio. Furthermore, we determined log2 ratio thresholds that minimized background noise while allowing for accurate SCNA calls.
Nexus 6.0 was first used for data visualization and analysis. Optimization of segmentation variables for use on WGA samples was determined with five-cell aliquots of normal female lymphocytes. The SNP-FASST2 segmentation algorithm on the basis of Hidden Markov Model was used. To normalize for genome-wide waviness because of changes in GC content, samples were corrected with the quadratic correction algorithm with a custom file that accounts for GC probe intensity surrounding each probe. Segmentation variables required a minimum of 10 probes and had a maximum segment size of 1 Mb, except for increasing the probe minimum to 25 for samples with an unusually high amount of interprobe variation. Probe re-centering was based on the genomic median of all probes or with the use of the ASCAT algorithm.19 Thresholds for calling CN alterations were as follows: high copy gain ≥0.41, copy gain ≥0.13, copy loss ≤−0.18, and high copy loss: ≤−0.9. Manufacturer-recommended settings were used for all other variables. Samples were manually curated to eliminate likely false-positive calls and to combine disjointed segments present in mosaic segments. Calls were eliminated if they were significant (P = 1.0), had log2 ratio values that were highly variable among probes or were not clearly different from surrounding normal regions, and did not have evidence of skewing in the B-allele frequency track.
For confirmation of Nexus CN results, data were also analyzed in R with the use of the Fused Lasso method (R package: cghFLasso) developed by Tibshirani and Wang.20 Raw segment data were imported into Excel (Microsoft, Redman, WA), and results were sorted on the basis of the number of probes per segment (n.mean) and the mean intensity of the segment (seg.mean). Segments with <10 probes or with a segment mean between −0.18 and 0.13 (normal or near-normal) were highlighted to be ignored. Data were then sorted on the basis of genomic coordinates and were curated to combine adjacent similar segments. Adjacent segments of similar seg.mean were combined, and an overall mean segment log2 ratio was calculated. Intervening segments that were previously ignored were included if they fit the segment value (ie, <10 probes if log2 ratio is normal, or if they have a segment n.mean value under the threshold of detection). A false discovery rate adjustment is applied for controlling the multiple CN alterations, and the significance threshold of 0.05 with adjusted FDR was used. Genomic regions that corresponded to SCNAs identified by Nexus were identified and used to create thresholds for identification of other possible undetected SCNAs. The overall background log2 ratio of diploid regions was calculated and used to define large mosaic CN alterations that have curated seg.mean values outside thresholds for detection. To identify possible segments detected by fused lasso, but missed by Nexus, data were re-sorted by seg.mean value, and any segments with >10 probes and segment mean within the range of those previously identified by Nexus were selected. New genomic regions were examined for probe distribution on Nexus, and segments identified by fused lasso that had evidence of being real (BAF skew, total shift in log2, few outlier probes) were included in analysis.
Validation and Quantitation of Copy Number Aberrations
Nanostring's nCounter Cancer CN panel (NanoString Technologies, Seattle, WA) was tested as a proof-of-principle analysis on 500 ng of WGA DNA from DTCs. This panel is designed to be used on unamplified bulk DNA with a 90% to 95% confidence with CN assessment on the basis of results from HapMap samples. A few modifications were incorporated to apply the assay on WGA DNA. We included five-cell aliquots from four normal male CD45+ lymphocytes, along with DTCs from prostate cancer patients, one matched metastatic tumor DNA amplified with WGA2, and one unmatched primary tumor DNA amplified with WGA2, in addition to the negative and positive controls provided by the manufacturer. Results were analyzed with invariant regions with known CN of 2 by SNP array data to normalize the CN quantification by the nCounter method. Raw probe values <25 were considered poor-performing probes and were filtered out before quantification analysis and comparison with SNP array results. Quantification output values were further classified with the following arbitrary cutoffs: CN values ≤1.3 were considered as true loss; CN values 1.4 to 1.6 as ambiguous loss; CN values ≥3.0 as true gain; and CN values 2.7 to 2.9 as ambiguous gain.
Real-time quantitative PCR assays to validate the CN status of PTEN and AR were designed on the basis of previously described methods.21, 22 One of the AR primers targeted exon 3, the location of the probes on the SNP array used on the DTCs. Additional control primers targeting regions known to be diploid in all samples were used for GPR15 and albumin genes (Table 1). The amplification efficiency of each primer was validated with a standard curve 10-fold dilution series (0.001 to 100 ng/μL) generated from similarly prepared WGA DNA derived from lymphocyte pools. Samples were prepared in duplicate with the Power SYBR Green Master Mix and the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). A melt curve analysis was completed at the end of each run to ensure that a single, clean product was being amplified. An R2 value of >0.98 was deemed acceptable for each primer pair amplification, although values were generally >0.99. Data were analyzed by ΔΔCt method for the generation of a value for CN.
Table 1.
Primer Sequences
| Primer name | Forward | Reverse |
|---|---|---|
| Albumin | 5′-TGTCATGCCCACACAAATCT-3′ | 5′-ATCCAAACTCATGGGAGCTG-3′ |
| GPR15 | 5′-TGTTCCTGACTGGAGTGCTG-3′ | 5′-CCAGAGAGGCAATGTGACAA-3′ |
| AR primer set 1 | 5′-GGGTTAGAAACAGGCATGGA-3′ | 5′-TTGCCTTGCTTTGACTTCCT-3′ |
| AR primer set 2 | 5′-CCATTGGGACTGTGCTAGGT-3′ | 5′-CTGGCTTCACCACTGACTGA-3′ |
| PTEN primer set 1 | 5′-AGGCTCCCAGACATGACAGCCATC-3′ | 5′-AAAGAGGAGCAGCCGCAGAAATGG-3′ |
| PTEN primer set 2 | 5′-ACACTACTGCTGTTTCCCTCTCCCT-3′ | 5′-ATGTGCCATCATTCTGGAACACAGC-3′ |
| GAPDH 100 bp | 5′-GTTCCAATATGATTCCACCC-3′ | 5′-CTCCTGGAAGATGGTGATGG-3′ |
| GAPDH 200 bp | 5′-AGGTGGAGCGAGGCTAGC-3′ | 5′-TTTTGCGGTGGAAATGTCCT-3′ |
| GAPDH 300 bp | 5′-AGGTGAGACATTCTTGCTGG-3′ | 5′-TCCACTAACCAGTCAGCGTC-3′ |
| GAPDH 400 bp | 5′-ACAGTCCATGCCATCACTGC-3′ | 5′-GCTTGACAAAGTGGTCGTTG-3′ |
Results
Optimization of WGA and SNP Array Processing for Limited Cell Numbers
To assess genomic alterations in rare cell populations that contained extremely low numbers (1 to 40) of cells, we first used normal human female lymphocytes and the human prostate cancer cell line LNCaP to establish the optimal methods for WGA and SNP array profiling.
WGA was performed in single tube reactions with the use of the PicoPlex WGA kit for Single Cells (Rubicon Genomics) on samples that contained up to 40 cells. For laser-captured microdissected tissue (containing ≥2000 cells) or other samples that contained low amounts of genomic DNA in the nanogram range, the WGA2 kit from Sigma-Aldrich yielded good SNP array results. Importantly, PicoPlex amplification yielded only template-specific amplification products, which allows for specific quantification of amplified DNA and detection of possible contamination within a water-only control reaction.
Extra caution to avoid the chance of PCR contamination is exercised during the WGA process. To avoid any possible loss of material, all genomic amplification steps were completed in the same tube in which cells were collected, and buffers were pipetted alongside the upper wall of the PCR tube to avoid making contact between the pipette tip and DNA-containing sample. Samples were mixed by low-speed vortex or flicking the tube to avoid using a pipette to mix samples.
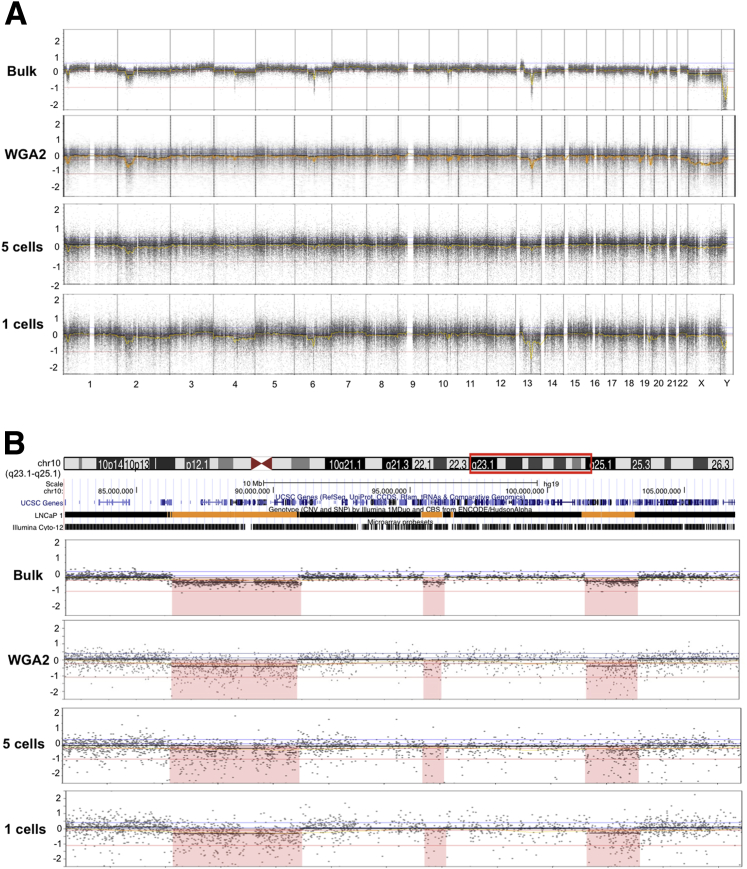
After extensive optimization, SNP array profiles from pools of 5- and 1-LNCaP cell showed high correlation to bulk gDNA (DNA extracted from cell pellet that contained approximately 2 × 106 cells) in terms of number, size, and location of SCNAs (Figure 1A and Supplemental Table S1). Similar to Hudson Alpha data (University of California Santa Cruz HAIB genome track), genomic profiling of bulk LNCaP genomic DNA identified 14 previously documented SCNAs, and an additional one CN gain and three CN-loss of heterozygosities.12, 13, 23 The schematic presentation of chromosome 10 (82,394,009 to 106,849,461 bp) in Figure 1B demonstrated the two deletions of 4.46 Mb and 1.94 Mb in size (the first and the third deletions in the region) were consistent between the samples although the ability to accurately detect SCNAs <1 Mb (the second deletion of 0.71 Mb) from one and five LNCaP cells was more variable. As an additional comparison, SNP array profiles of WGA LNCaP DNA obtained from 10 ng of starting material (equivalent to 1000 cells) showed essentially identical results. Of note, initial attempts to dilute high-quality genomic DNA to the quantity equivalent to 5 or 20 cells followed by WGA with either WGA2 or PicoPlex method yielded high levels of interprobe variability and poor detection of SCNAs. Thus, we used a micromanipulator to isolate replicate pools of 5, 10, 20, and 40 individual cells from a suspension and transferred these cells to sterile PCR tubes for subsequent WGA. We detail the optimization steps below.
Figure 1.
WGA and SNP array profiling on LNCaP populations of one and five- cells are reproducible and accurate. A: Representative images of LNCaP from bulk gDNA (DNA extracted from 2 × 106 cells), WGA2 (GenomePlex Complete Whole Genome Amplification kit)-amplified from 10 ng DNA, and Picoplex WGA amplified five- and one-cell aliquots of LNCaP. B: Schematic presentation of chromosome 10 (82,394,009 to 106,849,461 bp, red box) showing three deletions of 4.46 Mb, 0.71 Mb, and 1.94 Mb in size. The Hudson alpha track from University of California Santa Cruz genome browser was aligned to the corresponding regions. SNP, single nucleotide polymorphism; WGA, whole-genome amplification.
We first determined whether greater interprobe variation from five- and one-cell profiles is because of any systematic errors or biases introduced throughout the WGA process. We generated a reference library of 24 replicate pools that consisted of five-cell aliquots of normal female lymphocytes obtained from 10 unique individuals to create quality control measures for the WGA and SNP array processes that detected samples with good versus poor performance and then to determine the optimal computational settings for segmentation variables and thresholds to accurately and reproducibly detect SCNAs in rare cell populations.
Samples were examined for quality before hybridization to single nucleotide polymorphism–comparative genome hybridization arrays with the use of the following variables for acceptable range of quality before SNP array hybridization: i) ≥2.5 μg total DNA yield (or ≥45 ng/uL) after WGA; ii) a Gaussian distribution of WGA DNA fragments from 100 to 1000 bp via gel electrophoresis, with a peak range in 300 to 600 bp; and iii) proper amplification of 100-, 200-, 300-, and 400-bp bands from a multiplex GAPDH PCR reaction (data not shown). Samples that did not perform well in all three metrics were excluded from array hybridization.
After SNP array profiling, three variables were used to identify and exclude poor-performing samples from downstream analysis. These included call rate, Nexus's interprobe variance quality score, and the median log2 ratio values across the genome for each sample. On the basis of a panel of 24 NFRs, the following thresholds were used as cutoffs for sample analysis: call rate >75%, Nexus interprobe variance quality score <1.35, and median logR value >−0.25.
Assessing Reproducibility
We next verified the reproducibility of our methods with the use of replicate LNCaP samples, and replicate DTC samples from an individual with advanced-stage prostate cancer (2380C). Each sample was independently collected, amplified, and profiled by SNP array. The SCNA regions of five- and one-LNCaP cell were 99.97% and 62.60% identical to bulk LNCaP, respectively. The two replicate samples (20 cells/sample) of individuals 2380 showed almost identical SNP array profiles, with 96% of the copy number aberration regions in 2380C1 and 97% of the copy number aberration regions in 2380C2 shared with each other (Supplemental Table S2). Thus, our methods for WGA and SNP array profiling are highly efficient, reproducible, and capable of detecting SNCAs of 5 kb to 74 Mb in samples of 1 to 40 cells.
Sensitivity of Detection
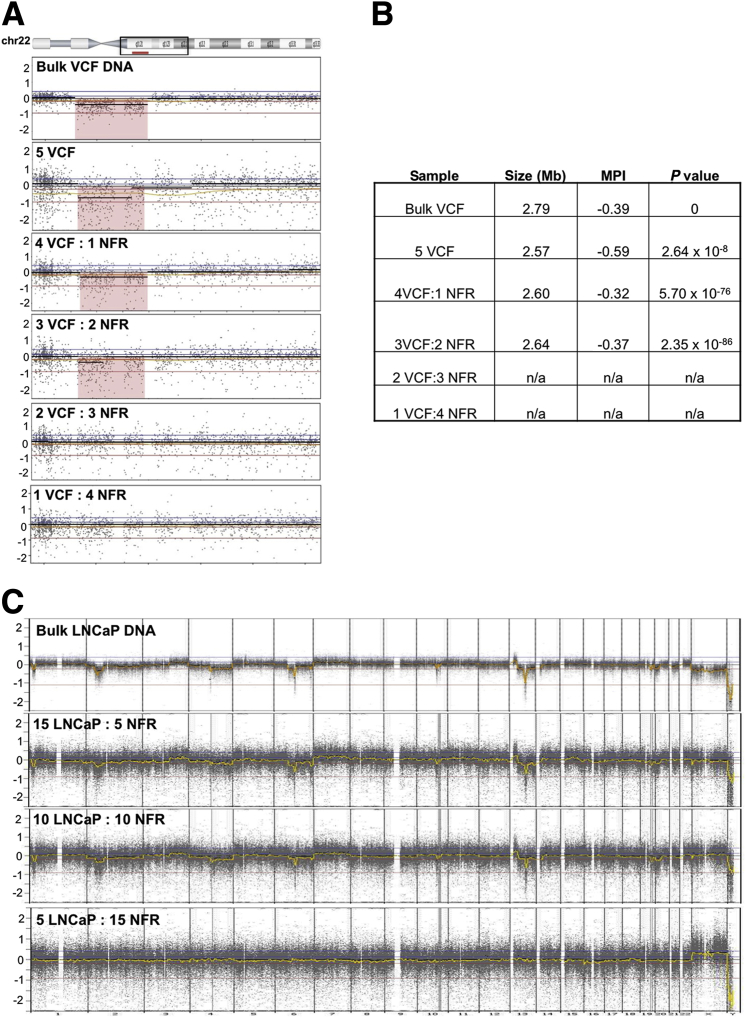
Given the heterogeneity of tumors and the likely mixture of genotypes present in patients' DTC populations, we performed cell line mixing experiments to determine the minimal prevalence of a given SCNA necessary for its detection. We generated mixtures of individual male cells with defined CN changes into NFRs to obtain pools of 5 and 20 cells with known frequencies of specific SCNAs. Male VCF syndrome cells, characterized by a 2.79-Mb deletion on 22q, were combined with female GM10473 lymphoblasts (Figure 2A). The 22q deletion was evident from the five pure VCF cells (100%), the mixture of four VCF cells with one NFR cell (80%), and the mixture of three VCF cells with two NFR cells (60%), but undetectable in the mixture of two VCF cells with three NFR cells (40%) and the 1VCF:4NFR mixture (20%). The size, magnitude, and P value of the deletion calls are shown in Figure 2B. Similarly, male LNCaP cells were mixed with female GM10966 lymphoblasts for profiling (Figure 2C). The SCNAs in bulk LNCaP DNA were seen in the combination of 15LNCaP:5NFR (75%) and 10LNCaP:10NFR (50%), but not in 5LNCaP:15NFR (25%). Collectively, these 5- and 20-cell mixtures indicated that our limit of detection (or sensitivity) for a heterozygous clone (ie, one allele) was between 40% and 50%. This was true for both gains and deletions, regardless of size (Figure 2).
Figure 2.
SNP array is able to detect SCNAs when present in ≥50% of the population. A: Results from 5-cell mixtures of a 22q-deletion syndrome (VCF) cell line and NFR cells. The VCF cells, characterized by a 2.79-Mb deletion on 22q, were combined with female GM10473 lymphoblasts. The 5VCF, 4VCF:1NFR, and 3VCF:2NFR mixtures all demonstrated visible deletion of 22q (shaded in pink), whereas no deletion was detected in the 2VCF:3NFR and 1VCF:4NFR mixtures. B: The size, MPI (representing the magnitude of deletion), and P value (significance) of deletion calls of the cell mixtures. C: Results from 20-cell mixtures of LNCaP cells with normal female GM10966 lymphoblasts. The SCNAs in bulk LNCaP DNA were seen in the combination of 15LNCaP:5NFR and 10LNCaP:10NFR mixtures but not in the 5LNCaP:15NFR mixture. MPI, mean probe intensity; NFR, normal female lymphoblast reference; SCNA, somatic copy number aberration; SNP, single nucleotide polymorphism; VCF, velocardiofacial
Genomic Profiling of DTCs
DTCs were isolated from bone marrow aspirates of patients with advanced stages of prostate cancer (Adv-DTC), including men with androgen-dependent metastases and others with metastatic castration-resistant prostate cancer.
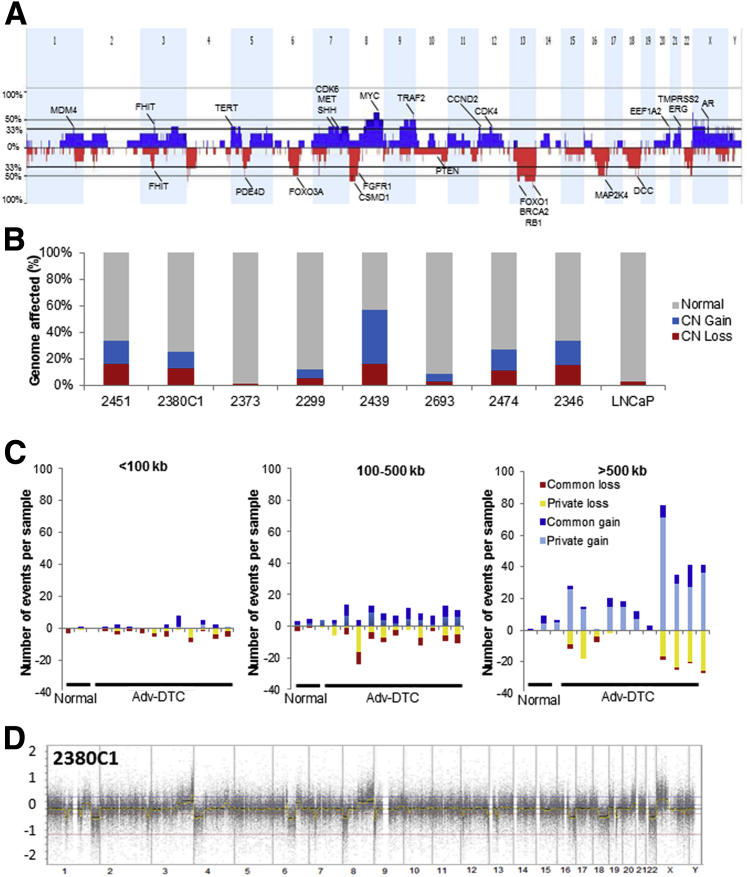
Genomic profiling on eight representative Adv-DTCs from eight different patients are summarized here. The combined genomic profile of the eight Adv-DTCs indicated multiple regions of CN gain or loss and included SCNAs characteristic of prostate cancer origin (Figure 3A). Overall, each Adv-DTC displayed 21.59 kb to 154.91 Mb CN gains and 2.99 kb to 135.37 Mb CN losses, with a median size of 13.97 Mb per SCNA. Aneuploidy was also common. The percentage of each genome (male = 3080 Mb) altered by SCNA is shown in Figure 3B. Most of these SCNAs, especially those >500 Kb in size, share <70% sequence overlap with common nucleotide variations, supporting that these are primarily cancer-specific SCNAs (Figure 3C). One representative DTC profiling (2380C1) is shown in Figure 3D.
Figure 3.
Genomic profiles of DTC from patients with advanced prostate cancer show highly aberrant genomes. A: Combined genome profile of the eight Adv-DTCs from eight different patients, with selected genes of interest highlighted. B: Individual genomic profile results for the eight patients. The x axis indicates the patient case number or cell line. y axis indicates the percentages of genome of each sample that is normal (gray), CN gain/amplification (blue), and CN loss (red). The cell numbers in the samples were 20 cells for patient 2451, 20 cells for patient 2380C1, 10 cells for patient 2373, 10 cells for patient 2299, 13 cells for patient 2439, 5 cells for patient 2693, 10 cells for patient 2474, and 20 cells for patient 2346. The bulk LNCaP DNA result is shown on the far right. C: The distribution of size and type of CNAs in DTCs relative to normal and tumor samples. Dark bars indicate CN variations that are common among the population, whereas yellow and light blue indicate CNAs that are considered private and likely to be tumor specific. Normals include CD45+ lymphocytes from the blood or bone marrow. D: A representative DTC profiling (2380C1) is shown. Adv, advanced; CN, copy number; CNA, copy number aberration; DTC, disseminated tumor cell.
The minimal overlapping regions (MORs) of SCNAs present in greater than three of eight samples (>37.5) are noted in Table 2. This list includes many chromosomal locations that were previously reported14 as frequent alterations in primary or metastatic prostate tumors, including CN gain of MYC (8q) and CN loss of BRCA2 (13q), FOXO1 (13q), PER1 (17p), RB1 (13q), TP53 (17p), and WWOX (16q). In addition, we detected novel MORs, including CN gain of GATA1 (Xp), KDM6A (Xp), and KDM5C (Xp) and CN loss of FGFR3 (4p), PPAP2A (5q), and KIF2A (5q). Some well-known prostate cancer-related genes, including AR (Xq), TMPRSS2 (21q), ERG (21q), and EZH2 (7q), were also detected with CN gain in MORs in three of eight samples (37.5%) (Supplemental Table S3).
Table 2.
Minimal Overlapping Regions of Somatic Copy Number Alterations in >3 of 8 Disseminated Tumor Cell Samples from Advanced Prostate Cancer
| Cytoband | Size (Mb) | Total genes | Genes of interest | Maximum frequency (%) |
|---|---|---|---|---|
| Gain | ||||
| 3p14.2 | 0.9 | 2 | FHIT, PTPRG | 50 |
| ∗8q13.2–q24.3 | 77.4 | 568 | TPD52, NBN, RUNX1T1, COX6C, EXT1, SLC25A32, MYC | 50 |
| ∗9q13–q34.3 | 70.0 | 1032 | MIR7-1, FANCC, PTCH1, XPA, FOXE1, NR4A3, TAL2, TNFSF8, SET, FNBP1, ABL1, NUP214, NOTCH1 | 50 |
| Xp22.33–p11.21 | 56.2 | 502 | IL3RA, CRLF2, P2RY8, KDM6A, SSX1, SSX4, WAS, GATA1, TFE3, SSX2, KDM5C | 50 |
| Loss | ||||
| 4p16.3–p14 | 38.1 | 313 | FGFR3 | 62.5 |
| 5q11.2–q13.2 | 15.4 | 106 | IL6ST, PID3R1,PPAP2A, KIF2A | 50 |
| ∗6q13–q21 | 41.4 | 256 | CD109, BACH2, PRDM1, FOXO3 | 50 |
| ∗8p23.3–p12 | 37.1 | 376 | PCM1, WRN | 62.5 |
| ∗13q13.1–q34 | 82.3 | 495 | LHFP, TTL, FOXO1, BRCA2, LCP1, RB1, ERCC5 | 62.5 |
| ∗16q13–q24.3 | 33.4 | 511 | WWOX, MMP2, CDH13, CDH1, MAF, CBFB, NQO1 | 62.5 |
| ∗17p13.1–p12 | 7.7 | 187 | TP53, PER1, GAS7, MAP2K4 | 50 |
| ∗18q12.1–q23 | 51.2 | 301 | SMAD4, MALT1, BCL2, KDSR | 50 |
| 22q13.2–q13.33 | 9.0 | 184 | MKL1, EP300 | 50 |
Minimal overlapping region >37.5% of samples (3 of 8), excluding regions with no coding genes.
Previously reported somatic copy number aberrations in disseminated tumor cells.14
Validation of Copy Number Aberrations
We validated the presence of SCNAs in the Adv-DTC population described in the section above with the use of two independent methods: Nanostring nCounter Cancer CN Assay and real-time quantitative PCR.
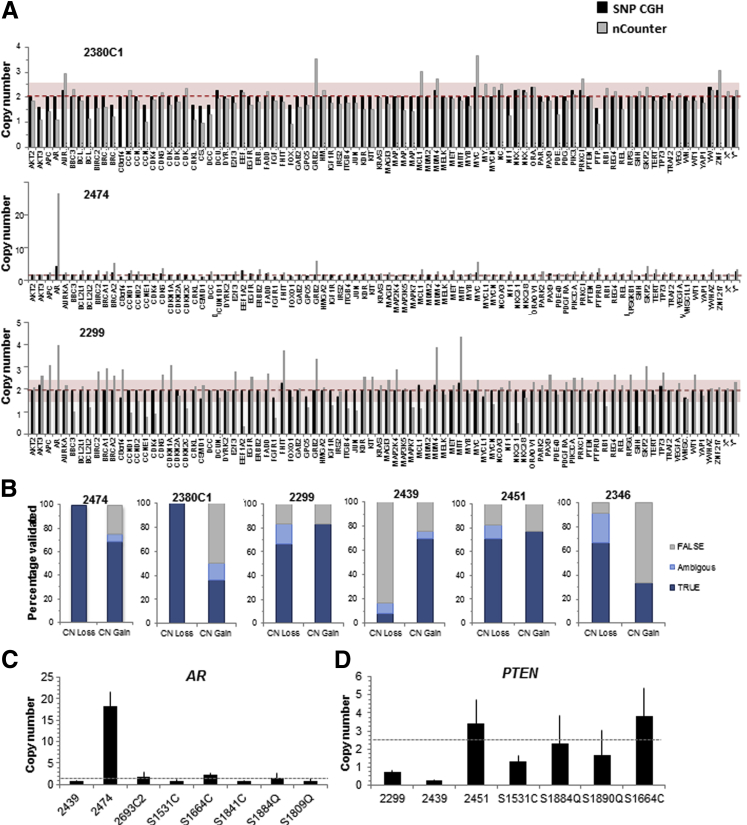
The nCounter assay is a non–amplification-based method for quantification of genomic CN in a multiplex fashion. Samples are normalized to normal controls with the use of invariant regions representing each chromosome. A total of 86 genes were included, each assayed by three unique probes. The results on WGA2-amplified products from 10 ng of starting DNA extracted from laser-captured microdissection of prostate cancer tissue showed results similar to unamplified bulk DNA (data not shown). The results on Picoplex-amplified products from DNA extracted from 10 to 20 Adv-DTCs are shown in Figure 4. Overall, there is a 70% to 80% concordance rate between the nCounter Assay results and the SNP array results (Figure 4A). Concordance is better for CN losses than for gains (Figure 4B).
Figure 4.
Validation of CN alterations in Adv-DTC. A: Representative Nanostring nCounter results for individuals 2380, 2474, and 2299. Red dashed line represents CN = 2 (normal) values in Illumina SNP-CGH, red faded box represents Nanostring CN = 1.5–2.5 normal range. B: Overall validation of CNAs detected in six individuals with the use of Nanostring nCounter assay (Loss: true = CN ≤ 1.4, Ambiguous = CN1.5–1.6, false = CN ≥ 1.7; Gain: true = CN ≥ 2.6, ambiguous = CN2.4–2.5, false = CN ≤ 2.3). C: Quantitation of AR amplification in 2474; other DTC and CD45+ samples do not have AR amplification. D: Quantitation of PTEN loss in 2299 and 2439; other DTC and CD45+ samples have normal PTEN CN. Dashed gray lines in B and C represent mean CN of nonaberrant samples. Adv, advanced; CGH, comparative genome hybridization; CN, copy number; CNA, copy number aberration; DTC, disseminated tumor cell; SNP, single-nuclear polymorphism.
We also established a highly sensitive and quantitative PCR-based validation assay that we used to quantify AR amplification in patient 2474 (Figure 4C) and to verify CN losses at the PTEN locus in patients 2299 and 2439 (Figure 4D). Patient 2474 had castration-resistant metastatic disease and showed a focal high-level amplification of the AR locus. Although SNP array analysis successfully detected this individual's high-level AR amplification, it was unable to provide a relative CN because of the nonlinear relation of CN and probe intensity for high-level amplifications. Control samples from CD45+ lymphocytes or from Adv-DTC without AR gain displayed a mean of 1.25 ± 0.41 copies of the AR locus per cell. In contrast, Adv-DTC from individual 2474 had 18.4 ± 3.2 copies of the AR locus per cell (Figure 4C). These results correlated well with preliminary data obtained from whole-genome sequencing, which quantified 23 AR copies per cell for this sample (data not shown). For the PTEN locus, two Adv-DTC samples from patients 2299 and 2439, who had lethal prostate cancer, had evidence of a mosaic loss of the entire chromosome 10 by SNP array. We validated loss of PTEN in these two individuals, quantifying 0.73 ± 0.09 and 0.27 ± 0.04 copies of PTEN for individuals 2299 and 2439, respectively (Figure 4D). Control samples from CD45+ lymphocytes or from Adv-DTC without evidence of PTEN loss had mean of 2.51 ± 1.09 copies of PTEN.
Comparison of DTC with Matched Metastatic Tumors
Two individuals had multiple matched metastatic tumor samples available, which were obtained simultaneously with the Adv-DTCs at the time of death. Genomic profiles of the matched tumors were compared with that of the Adv-DTCs.
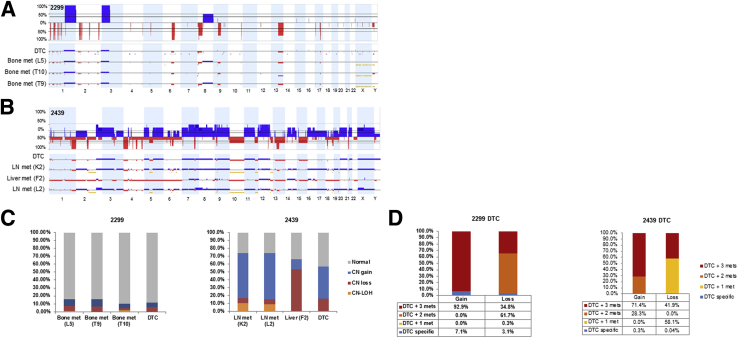
Individual 2299 was a 77-year-old patient with metastatic castration-resistant prostate cancer initially diagnosed with prostate cancer of Gleason score 10, with a PSA of 12.0 ng/mL, which rose to 762.3 ng/mL at the time of death. SNP array profiling of his DTCs revealed several genomic aberrations, which appear to be mosaic, including losses at 6q, 8p; gains at 1q, 3q, and 8q (Figure 5A). Three matched bone metastases from vertebrae L5, T9, and T10 were available for SNP array analysis (Figure 5, A and C). Tumors from L5 and T9 had nearly identical genomic profiles, with the notable exception that T9 tumor had higher magnitude of SCNAs, indicative of a greater percentage of the sample being tumor. T10 tumor had less shared events with L5 and L9, and the magnitude of copy number aberrations in T10 is generally lower, indicating the presence of a significant proportion of cells with a normal genotype (Supplemental Tables S4 and S5). Of the genomic alterations measured in the DTCs, approximate 7% and 3% of the copy gains and copy losses, respectively, were not observed in the metastatic tumors sampled (Figure 5D).
Figure 5.
Comparison of matched DTC and metastatic tumors from two individuals supports their relation. A: SNP array whole genome plot of DTC 2299 and three patient-matched bone mets; blue represents CN gains and red represents loss. B: SNP array data of DTC 2439 and three patient-matched soft tissue mets. Yellow horizontal bars in samples denote regions ≥3 Mb that include copy-neutral LOH . C: Comparison of the percentage of each genome affected by SCNAs and CN LOH (note, CN LOH could not be determined for DTCs). D: Percentage of SCNAs in DTC 2299 and DTC 2439 that are also detected in the matched metastatic tumors. CN, copy number; DTC, disseminated tumor cell; LN, lymph node; LOH, loss of heterozygosity; mets, metastasis; SCNA, somatic copy number aberrations; SNP, single-nuclear polymorphism.
Individual 2439 was a 76-year-old castration-resistant prostate cancer patient initially diagnosed with prostate cancer of Gleason score 6 and a PSA of 7.0 ng/mL, which rose to 3700 ng/mL near the time of death, 16 years after initial diagnosis and prostatectomy. In addition to DTCs, three metastatic tumors from lymph nodes and liver were available for SNP array analysis (Figure 5B). In contrast to patient 2299, patient 2439 had a high degree of correlation between his DTC and visceral metastases. Only 0.3% and 0.04% of SCNAs gain and loss regions, respectively, of DTC population were DTC specific (Figure 5D). However, the metastases had several genomic aberrations that were not detected in the DTC population. Notably, >85% of the SCNA loss regions of the liver metastasis were not shared with the two lymph node metastases. The two lymph node metastases were nearly identical and shared approximate 80% of SCNA gain regions that were not detected in the liver metastasis (Supplemental Tables S4 and S5). The SCNAs present in metastases, but not detected in DTCs, may either be specific to the metastases or present at low levels in the DTC population below the detection limit of 50%.
Discussion
The presence of DTCs in the bone marrow of prostate cancer patients has important prognostic and therapeutic implications.6, 24, 25 DTCs can be detected in patients with clinically dormant disease for decades before the development of overt metastatic disease. Bone marrow and blood offer an opportunity for repeated sampling and represent an alternative to biopsies of metastatic sites, which have shown an unsatisfactorily low successful rate, particularly of bone metastasis. Studying the genomics of CTC or DTC is of great interest to understand cancer biology, including heterogeneity, to discover new noninvasive biomarkers, and to find novel therapeutic targets. However, the rarity of CTC/DTCs poses substantial challenges for their isolation and molecular characterization. The heterogeneity of CTC/DTCs further demands better refinement of methods to delineate the genetic complexity of these cells. This study described important progress toward a robust and consistent method to reproducibly profile DTCs with high-resolution SNP array platforms.
The goals of methods development component of this study were to ensure that, when applied to ≤40 cells, WGA and SNP array profiling produce i) an acceptable dynamic range to detect SCNAs, ii) high reproducibility across replicates, and iii) consistent, low levels of background. We demonstrated in this study the achievement of these goals, which set the stage for systematic characterization of DTCs and their role in prostate cancer metastasis.
Applying the refined methods for DTC genomic analysis provided a reproducible and sensitive assay for studies of DTCs derived from men with advanced prostate cancer. Our group and others have shown previously that DTCs (and CTCs) from patients with advanced-stage diseases exhibit a large number of genomic aberrations similar to those seen in metastatic tumors.12 DTCs in these patients are frequently characterized by gain of chromosome 7 and 8q and losses in 8p, 12p23, 10q26, 13q, and 16q21, which are consistent with those found in metastatic prostate cancers. With the high-resolution SNP arrays, we not only confirmed these findings but were also able to define MORs that likely contain critical genes important for prostate cancer tumorigenesis and metastasis (Table 2 and Figure 3A). Among the most frequently gained regions, the MYC oncogene is well documented, as are AR gain and TMPRSS2/ERG alterations. Other gained regions, including MDM4, TERT, EZH2, TRAF, CDK4, etc. (Figure 3A), are new findings or recently described in prostate cancer. Among the most commonly deleted regions, RB1 and FOXO1 are relatively sparsely documented. Notably, six of the eight Adv-DTC samples we analyzed demonstrated FOXO1 gene deletion (Figure 3A and Table 2). The forkhead box protein family members are transcription factors that bind to AR and regulate its association with androgen response elements.26 FOXO proteins have tumor suppressor activity.27 Deletion of FOXO1 on 13q14 was observed in approximately one-third of prostate cancer cell lines, primary tumors, and xenografts.28 Loss of FOXO1 increases the basal activity of AR and sensitizes it to lower androgen levels or other nonandrogenic ligands.29 FOXO1 also inhibits Runx2 transcription activity and prostate cancer cell migration and invasion.30
Multiple matched tumor samples along with DTCs from the same patient are extremely difficult to obtain. In two patients whose DTCs and matching metastatic tumors from several different organ sites were analyzed and compared (Figure 5), the finding that the DTC samples did not exhibit a subset of SCNAs that were observed in the matching metastatic tumors suggested that DTCs may represent an earlier precursor to the metastatic tumors which developed additional mutations, or that some DTC populations with admixtures of non-DTCs lowered the detection of certain genomic aberrations. The results also reflected the heterogeneity of the metastases, which may be influenced by the microenvironment of the various metastatic sites (Figure 5C). In addition, a small portion of the DTC SCNAs (<10%) was not observed in the metastatic tumors sampled (Figure 5D), further supporting the heterogeneity of the DTC population. Overall, the SCNAs detected in DTCs reflected the patients' metastatic disease.
Two models currently exist for the dissemination of prostate tumor cells: one is the linear progression model, which hypothesizes a late dissemination that does not happen until enough genetic abnormalities have accumulated in a step-wise fashion associated with disease progression under selection for competitive fitness, and the parallel progression model, which proposes an early dissemination followed by parallel progression of the individual tumor at the primary prostate and the metastatic sites.31 Our data support the latter and argue that tumor cells are released at an early stage of cancer progression. It remains to be elucidated how DTCs enter and exit dormancy and lead to frank metastasis. The technical progress demonstrated in this study that allowed for genetic profiling of as few as one cell will contribute to future endeavors to understand the mechanisms of prostate cancer metastasis.
Acknowledgments
We thank Dr. Barbara Trask (Member Emeritus, Fred Hutchinson Cancer Research Center) for tremendous support, Dr. Li Hsu and Roman Gulati for statistical consultation, Cassie Sather for performing the SNP array hybridization, and Dr. Gary Geiss for performing the nCounter Assay. We thank the patients and their families who were willing to participate in the Prostate Cancer Donor Program and the investigators Drs. Celestia Higano, Bruce Montgomery, Evan Yu, Martine Roudier, and Lawrence True for their contributions to the University of Washington Medical Center Prostate Cancer Donor Rapid Autopsy Program.
Footnotes
Supported by the National Cancer Institute grants P01CA085859 (R.L.V.; sub project M.F.) and P01 CA163227 (P.S.N.), the Pacific Northwest Prostate Cancer SPORE grant P50CA97186 (P.S.N.), the Prostate Cancer Foundation (R.L.V.), and the Richard M. Lucas Foundation (R.L.V.).
Y.W. and J.R.S. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.jmoldx.2015.08.004.
Supplemental Data
References
- 1.Crowley E., Di Nicolantonio F., Loupakis F., Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472–484. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 2.de Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W., Pienta K.J., Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 3.Meng S., Tripathy D., Frenkel E.P., Shete S., Naftalis E.Z., Huth J.F., Beitsch P.D., Leitch M., Hoover S., Euhus D., Haley B., Morrison L., Fleming T.P., Herlyn D., Terstappen L.W., Fehm T., Tucker T.F., Lane N., Wang J., Uhr J.W. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 4.Sosa M.S., Bragado P., Aguirre-Ghiso J.A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weckermann D., Polzer B., Ragg T., Blana A., Schlimok G., Arnholdt H., Bertz S., Harzmann R., Klein C.A. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol. 2009;27:1549–1556. doi: 10.1200/JCO.2008.17.0563. [DOI] [PubMed] [Google Scholar]
- 6.Morgan T.M., Lange P.H., Porter M.P., Lin D.W., Ellis W.J., Gallaher I.S., Vessella R.L. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15:677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stott S.L., Lee R.J., Nagrath S., Yu M., Miyamoto D.T., Ulkus L., Inserra E.J., Ulman M., Springer S., Nakamura Z., Moore A.L., Tsukrov D.I., Kempner M.E., Dahl D.M., Wu C.L., Iafrate A.J., Smith M.R., Tompkins R.G., Sequist L.V., Toner M., Haber D.A., Maheswaran S. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2:25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma X., Xiao Z., Li X., Wang F., Zhang J., Zhou R., Wang J., Liu L. Prognostic role of circulating tumor cells and disseminated tumor cells in patients with prostate cancer: a systematic review and meta-analysis. Tumour Biol. 2014;35:5551–5560. doi: 10.1007/s13277-014-1731-5. [DOI] [PubMed] [Google Scholar]
- 9.Schilling D., Todenhofer T., Hennenlotter J., Schwentner C., Fehm T., Stenzl A. Isolated, disseminated and circulating tumour cells in prostate cancer. Nat Rev Urol. 2012;9:448–463. doi: 10.1038/nrurol.2012.136. [DOI] [PubMed] [Google Scholar]
- 10.Zong C., Lu S., Chapman A.R., Xie X.S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science. 2012;338:1622–1626. doi: 10.1126/science.1229164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohr J.G., Adalsteinsson V.A., Cibulskis K., Choudhury A.D., Rosenberg M., Cruz-Gordillo P. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014;32:479–484. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holcomb I.N., Grove D.I., Kinnunen M., Friedman C.L., Gallaher I.S., Morgan T.M., Sather C.L., Delrow J.J., Nelson P.S., Lange P.H., Ellis W.J., True L.D., Young J.M., Hsu L., Trask B.J., Vessella R.L. Genomic alterations indicate tumor origin and varied metastatic potential of disseminated cells from prostate cancer patients. Cancer Res. 2008;68:5599–5608. doi: 10.1158/0008-5472.CAN-08-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holcomb I.N., Young J.M., Coleman I.M., Salari K., Grove D.I., Hsu L., True L.D., Roudier M.P., Morrissey C.M., Higano C.S., Nelson P.S., Vessella R.L., Trask B.J. Comparative analyses of chromosome alterations in soft-tissue metastases within and across patients with castration-resistant prostate cancer. Cancer Res. 2009;69:7793–7802. doi: 10.1158/0008-5472.CAN-08-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenborn J.R., Nelson P., Fang M. Genomic profiling defines subtypes of prostate cancer with the potential for therapeutic stratification. Clin Cancer Res. 2013;19:4058–4066. doi: 10.1158/1078-0432.CCR-12-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heitzer E., Auer M., Gasch C., Pichler M., Ulz P., Hoffmann E.M., Lax S., Waldispuehl-Geigl J., Mauermann O., Lackner C., Hofler G., Eisner F., Sill H., Samonigg H., Pantel K., Riethdorf S., Bauernhofer T., Geigl J.B., Speicher M.R. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 2013;73:2965–2975. doi: 10.1158/0008-5472.CAN-12-4140. [DOI] [PubMed] [Google Scholar]
- 16.Trask B., van den Engh G., Nussbaum R., Schwartz C., Gray J. Quantification of the DNA content of structurally abnormal X chromosomes and X chromosome aneuploidy using high resolution bivariate flow karyotyping. Cytometry. 1990;11:184–195. doi: 10.1002/cyto.990110121. [DOI] [PubMed] [Google Scholar]
- 17.Morrissey C., True L.D., Roudier M.P., Coleman I.M., Hawley S., Nelson P.S., Coleman R., Wang Y.C., Corey E., Lange P.H., Higano C.S., Vessella R.L. Differential expression of angiogenesis associated genes in prostate cancer bone, liver and lymph node metastases. Clin Exp Metastasis. 2008;25:377–388. doi: 10.1007/s10585-007-9116-4. [DOI] [PubMed] [Google Scholar]
- 18.Ellis W.J., Pfitzenmaier J., Colli J., Arfman E., Lange P.H., Vessella R.L. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology. 2003;61:277–281. doi: 10.1016/s0090-4295(02)02291-4. [DOI] [PubMed] [Google Scholar]
- 19.Van Loo P., Nordgard S.H., Lingjaerde O.C., Russnes H.G., Rye I.H., Sun W., Weigman V.J., Marynen P., Zetterberg A., Naume B., Perou C.M., Borresen-Dale A.L., Kristensen V.N. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci U S A. 2010;107:16910–16915. doi: 10.1073/pnas.1009843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibshirani R., Wang P. Spatial smoothing and hot spot detection for CGH data using the fused lasso. Biostatistics. 2008;9:18–29. doi: 10.1093/biostatistics/kxm013. [DOI] [PubMed] [Google Scholar]
- 21.D'Haene B., Vandesompele J., Hellemans J. Accurate and objective copy number profiling using real-time quantitative PCR. Methods. 2010;50:262–270. doi: 10.1016/j.ymeth.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Weksberg R., Hughes S., Moldovan L., Bassett A.S., Chow E.W., Squire J.A. A method for accurate detection of genomic microdeletions using real-time quantitative PCR. BMC Genomics. 2005;6:180. doi: 10.1186/1471-2164-6-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu X., Randhawa G., Friedman C., O'Hara-Larrivee S., Kroeger K., Dumpit R., True L., Vakar-Lopez F., Porter C., Vessella R., Nelson P., Fang M. A novel four-color fluorescence in situ hybridization assay for the detection of TMPRSS2 and ERG rearrangements in prostate cancer. Cancer Genet. 2013;206:1–11. doi: 10.1016/j.cancergen.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruck S., Gakis G., Stenzl A. Circulating and disseminated tumor cells in the management of advanced prostate cancer. Adv Urol. 2012;2012:135281. doi: 10.1155/2012/135281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lilleby W., Stensvold A., Mills I.G., Nesland J.M. Disseminated tumor cells and their prognostic significance in nonmetastatic prostate cancer patients. Int J Cancer. 2013;133:149–155. doi: 10.1002/ijc.28002. [DOI] [PubMed] [Google Scholar]
- 26.Grasso C.S., Wu Y.M., Robinson D.R., Cao X., Dhanasekaran S.M., Khan A.P., Quist M.J., Jing X., Lonigro R.J., Brenner J.C., Asangani I.A., Ateeq B., Chun S.Y., Siddiqui J., Sam L., Anstett M., Mehra R., Prensner J.R., Palanisamy N., Ryslik G.A., Vandin F., Raphael B.J., Kunju L.P., Rhodes D.R., Pienta K.J., Chinnaiyan A.M., Tomlins S.A. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang D.C., Palmer D.A., Zarei M., Shah P., Folsom C., Beyth R.J., Stoffs T.L., Neuberger M.M., Dahm P. A systematic review of the quality of evidence of ablative therapy for small renal masses. J Urol. 2012;187:44–47. doi: 10.1016/j.juro.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 28.Dong X.Y., Chen C., Sun X., Guo P., Vessella R.L., Wang R.X., Chung L.W., Zhou W., Dong J.T. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 29.Liu P., Li S., Gan L., Kao T.P., Huang H. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res. 2008;68:10290–10299. doi: 10.1158/0008-5472.CAN-08-2038. [DOI] [PubMed] [Google Scholar]
- 30.Renault V.M., Thekkat P.U., Hoang K.L., White J.L., Brady C.A., Kenzelmann Broz D., Venturelli O.S., Johnson T.M., Oskoui P.R., Xuan Z., Santo E.E., Zhang M.Q., Vogel H., Attardi L.D., Brunet A. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene. 2011;30:3207–3221. doi: 10.1038/onc.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joosse S.A., Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res. 2013;73:8–11. doi: 10.1158/0008-5472.CAN-12-3422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.