Abstract
The role of exogenously added methyl jasmonate (MeJA), a lipid-derived signaling compound, in inducing oxidative stress in the marine red macroalga Gracilaria dura was investigated. MeJA at a concentration of 1–100 µM was a strong stimulant of reactive oxygen species (H2O2, HO· and O2·−) (P < 0.05) causing considerable oxidative stress in G. dura. This further led to lipid peroxidation and degradation of the pigments Chl a and phycocyanin, with a concomitant increase in phycoerythrin. The MeJA-induced oxidative burst also led to the induction of a fatty acid oxidation cascade, resulting in the synthesis of hydroxy-oxylipins and the up-regulation of the 13-lipoxygenase pathway. Electrospray ionization-mass spectrometry-based shotgun lipidomic analysis revealed that monogalactosyldiacylglycerol (a chloroplastic glycerolipid) and phosphatidylcholine (extrachloroplastidic phopholipid) were the most affected lipid classes. The degradation of 18:3-fatty acid-containing monogalactosyldiacylglycerol inferred that it provided fatty acyl chains for the biosynthesis of 13-hydroperoxylinolenic acid, which was further directed towards either the jasmonate pathway or other alternative pathways of the fatty acid oxidation cascade, analogous to higher plants. Also, G. dura modulated the lipid acyl chains in such a way that no significant change was observed in the fatty acid profile of the treated thalli as compared with those of the control, except for C16:0, C16:1 (n-9), C20:3 (n-6) and C20:4 (n-6) (P < 0.05). Furthermore, MeJA caused the accumulation of phenolic compounds and the up-regulation of enzymes involved in secondary metabolism such as polyphenol oxidase, shikimate dehydrogenase and phenylalanine ammonia-lyase, indicating a shift towards secondary metabolism as a defense strategy to combat the induced oxidative stress.
Keywords: Gracilaria dura, Lipidomics, Methyl jasmonate, Oxylipins, ROS, Stress
Introduction
Jasmonates are lipid-derived signal molecules that mediate a plethora of biological processes from stress and defense responses to reproductive development, secondary metabolism and senescence in plants (Browse 2009, Wasternack et al. 2012). They are generated via the allene oxide synthase (AOS) branch of the lipoxygenase (LOX) pathway of lipid oxidation and exert their effects by orchestrating large-scale reprogramming of gene expression (Kombrink 2012).
Methyl jasmonate (MeJA) is one of the most active forms of jasmonic acid (JA) in plants. It is produced by methyl ester formation on C1 of JA by JA-specific methyl transferase (Seo et al. 2001). The presence of JA and MeJA has been reported in several lineages of non-vascular plants (Hamberg and Gardner 1992), including unicellular green algae (Fujii et al. 1997), euglenophytes (Ueda et al. 1991) and the rhodophyte Gelidium latifolium (Krupina and Dathe 1991). The entire set of enzymes necessary for the biosynthesis of JA from α-linolenic acid (ALA) has been identified in marine red algae such as Gracilariopsis sp. (Hamberg and Gerwick 1993) and Lithothamnion corallioides (Hamberg 1992). Recently, the genome sequence of the brown alga Ectocarpus siliculosus (Cock et al. 2010) also showed the candidate genes for AOS which catalyzes the initial step of JA biosynthesis, and allene oxide cyclase (AOC). In contrast, no candidate genes could be identified for AOS and AOC in the genome of the red alga Chondrus crispus which contained two candidate genes for 12-oxo-phytodienoic acid reductase (12-OPR) and genes involved in β-oxidation within the JA biosynthetic pathway (Collén et al. 2013). Moreover, no genes for jasmonic acid carboxyl methyl transferase have been identified in the genome of both the macroalgae known so far, E. siliculosus and C. crispus, indicating that an enzyme different from those characterized in land plants may be present in these macroalgae (Cock et al. 2010, Collén et al. 2013). Although the occurrence and relevance of JA/MeJA in macroalgae is puzzling, a few known studies have implicated their roles in defense against biotic/abiotic stresses in C. crispus (Bouarab et al. 2004, Collén et al. 2007, Gaquerel et al. 2007), Fucus vesiculosus (Arnold et al. 2001) and Laminaria digitata (Küpper et al. 2009). However, it is unclear if MeJA is an endogenous compound in these algae. MeJA has only been detected in cell-free extracts of C. crispus after the addition of linolenic acid, and the attempts to identify JA in C. crispus cell homogenates have remained unsuccessful (Bouarab et al. 2004). Wiesemeier et al. (2008) also did not detect JA/MeJA and their biosynthetic precursor, 12-oxo-phytodienoic acid (12-OPDA), in the brown algal genera Colpomenia, Dictyota, Ectocarpus, Fucus, Himanthalia, Saccharina and Sargassum. In addition, treatment with ecologically relevant concentrations of JA and MeJA did not lead to a significant change in the profile of medium- and non-polar metabolites of the tested algae. A metabolic response of unspecific stress was observed only after the application of higher concentrations of these phytohormones (≥500 µg ml–1 medium; Wiesemeier et al. 2008). In contrast, Ritter et al. (2008, 2014) detected 12-OPDA in response to copper stress in L. digitata and E. siliculosus, indicating the occurrence of plant-like octadecanoid metabolites to regulate protective mechanisms towards copper stress.
Our understanding of jasmonates in macroalgae is limited to only these few reports, and the role of jasmonates in other macroalgae is largely unexplored. Thus, we studied the effect of MeJA on lipids, fatty acids (FAs) and oxylipin profiles in the red macroalga Gracilaria dura. We addressed the following key issues. (i) Does MeJA trigger lipid peroxidation and oxidative stress responses in G. dura? (ii) What are the lipidomic and hydroxy-oxylipin changes in response to MeJA in G. dura? (iii) Does MeJA instigate any effect on phenolic compounds accumulation and enzymes involved in secondary metabolism in G. dura?
Results
Lipid peroxidation, reactive oxygen species (ROS) production and in situ localization
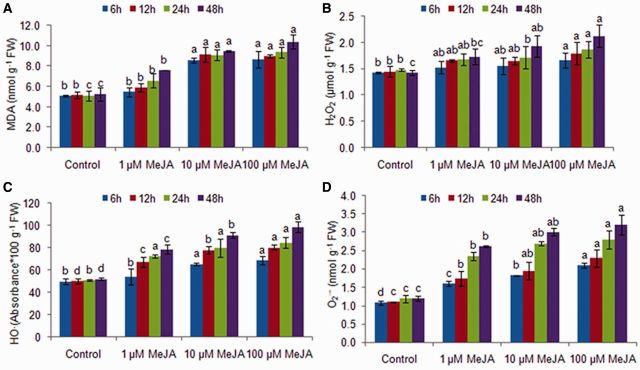
MeJA-treated G. dura thalli showed a dose- and time-dependent increase in lipoperoxide (thiobarbituric acid-reactive substances–malondialdehyde; TBARS–MDA) levels (1.1- to 2.0-fold) as compared with controls (P < 0.05) (Fig. 1). Further, the treated thalli showed an increase in hydrogen peroxide (H2O2) content (1.1- to 1.5-fold) with an increase in exogenous MeJA concentration and treatment duration (1.1- to 1.3-fold) (Fig. 1). Hydroxyl (HO·) and superoxide (O2·−) radicals also showed a dose- (1.1- to 1.9-fold and 1.1- to 1.7-fold, respectively) and time-dependent (1.2- to 1.4-fold and 1.5- to 3.2-fold, respectively) increase in the treated thalli as compared with the controls (Fig. 1). However, the highest rate of increase in H2O2 and HO· was observed at 12 h, then the rate of increase in their contents slowed down and again increased after 24 h, while the highest rate of increase in superoxide radical (O2·−) was observed at 24 h. These results indicated that the generation of O2·− radicals precedes the H2O2 and HO· bursts. Further, histochemical staining for the detection of in situ accumulation of H2O2 and O2·− using nitroblue tetrazolium (NBT) and 3,3′-diaminobenzidine (DAB), respectively, confirmed the MeJA-induced ROS production (Fig. 2). A blue formazone product formed by the reduction of NBT by O2·− clearly showed the generation of a superoxide radical that appeared first in the epidermal cells, then gradually progressed to the cortical cells and later was distributed all over the tissue. Similarly, the formation of H2O2-dependent brown precipitates was contingent on the exposure duration and the MeJA dose. Moreover, two-way analysis of variance (ANOVA) revealed that even though ROS production and lipid peroxidation observed in the MeJA thalli significantly increased with an increase in the MeJA dose and treatment duration (P < 0.05), these increases were independent of each other and the interaction of concentration and time was significant only for HO· and O2·− (Supplementary Table S1). Further, G. dura thalli at 48 h showed the signs of bleaching due to increased oxidative stress, and thus the experimental period was limited to 24 h for the study of other biochemical and lipidomic responses.
Fig. 1.
TBARS–MDA level and reactive oxygen species contents in methyl jasmonate-treated Gracilaria dura thalli (A) TBARS–MDA, (B) H2O2, (C) HO· and (D) O2·−. Different letters on the same shaded columns indicate that mean values for the particular incubation time were significantly different at P < 0.05.
Fig. 2.
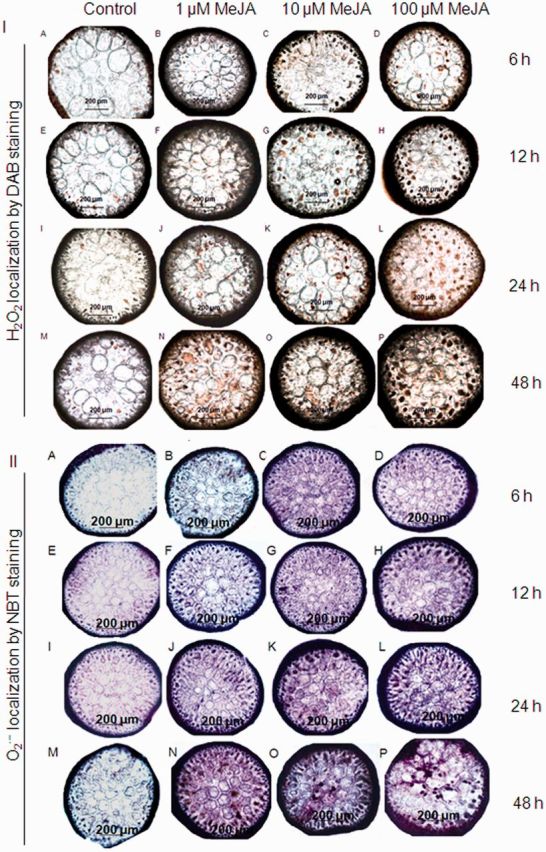
ROS generation in Gracilaria dura thalli treated with methyl jasmonate: (I) H2O2 by DAB staining and (II) O2·− by NBT staining.
Pigments
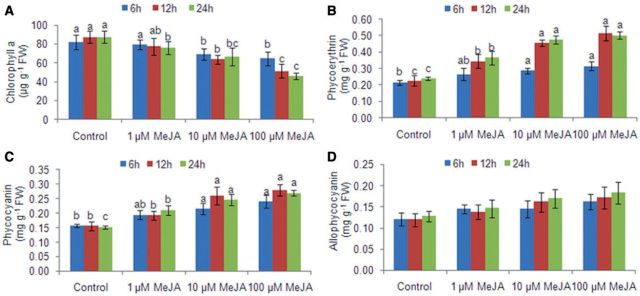
Chl a content decreased in the treated thalli as compared with the control thalli by 1.03- to 2.0-fold with an increase in the MeJA dose and by 1.02- to 1.4-fold with treatment time, due to increased ROS generation (Fig. 3). This decrease in Chl content was accompanied by an increase in phycobiliproteins, mainly phycoerythrin (1.2- to 2.1-fold) (P < 0.05). Phycocyanin and allophycocyanin showed only a dose-dependent increase of 1.2- to 1.8-fold and 1.1- to 1.4-fold, respectively, in the treated thalli (Fig. 3; Supplementary Table S1) Further, 1 µM MeJA-treated thalli showed a constant marginal increase in phycocyanin content (1.02- to 1.1-fold) while 10 and 100 µM MeJA-treated thalli showed an initial increase of 1.2-fold until 12 h and then slightly decreased. This maybe due to increased oxidative damage.
Fig. 3.
Effect of methyl jasmonate on (A) Chl a and phycobiliproteins, (B) phycoerythrin, (C) phycocyanin and (D) allophycocyanin in MeJA-treated Gracilaria dura. Different letters on the same shaded columns indicate that mean values for different MeJA concentrations were significantly different at P < 0.05 for each incubation time.
Lipids and fatty acids
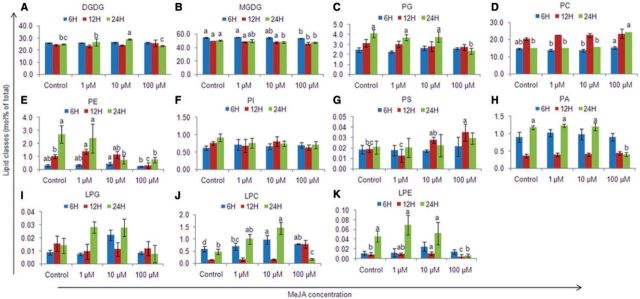
Total lipid content increased by 1.2- to 1.5-fold in the treated thalli as compared with the controls (P < 0.05). The thalli treated with 1 µM MeJA showed a constant increase in lipid content during the entire study period, while 10 and 100 µM MeJA-treated thalli showed an initial increase in the lipid content until 12 h but thereafter the total lipid content decreased at 24 h. Lipidomic analysis revealed that monogalactosyldiacylglycerol (MGDG) was the dominant lipid class, followed by digalactosyldiacylglycerol (DGDG), phosphatidylcholine (PC) and phosphatidylglycerol (PG), in both the control and treated thalli. Phosphatidylinositol (PI), phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidic acid (PA) were present as minor lipids (Fig. 4; Supplementary Fig. S1). Furthermore, there was a predominance of C36 (36:4) and C34 (34:1) acyl carbons in the chloroplastic lipids DGDG, MGDG and PG, indicating the presence of 18:2/18:2 or 18:1/18:0 acyl chains (Supplementary Table S2; Supplementary Figs. S2, S3). In addition, MGDG also contained C40 carbons (40:8 and 40:9) which together represented 13.8–27.8% of the total MGDGs, indicating the presence of 20:4/20:4 or 20:4/20:5 acyl chains. The extraplastidic phospholipids PC, PE, PI and PA were dominated by C40 acyl carbons (mainly 40:8 or 40:7) and represented 60–68% of total PC, 36.6–43.7% of total PE, 35.4–45.4% of total PI and 23.2–58% of total PA; in the case of PS, C36 acyl carbons were dominant, followed by C40 acyl carbons (Supplementary Table S2; Supplementary Figs. S4–S6).
Fig. 4.
Lipid class composition of Gracilaria dura thalli treated with methyl jasmonate. (A) Digalactosyldiacylglycerol (DGDG), (B) monogalactosyldiacylglycerol (MGDG), (C) phosphatidylglycerol (PG), (D) phosphatidylcholine (PC), (E) phosphatidylethanolamine, (F) phosphatidylinositol (PI), (G) phosphatidylserine (PS), (H) phosphatidic acid, (I) lyso-phosphatidylglycerol (LPG), (J) lyso-phosphatidylcholine (LPC) and (K) lyso-phosphatidylethanolamine (LPE). Different letters on the same shaded columns indicate that mean values for different MeJA concentrations were significantly different at P < 0.05 for each incubation time.
MGDG, PG, PC, PE and PA showed significant dose- and time-dependent changes with respect to the control, and the interaction effects of exogenous MeJA concentration and treatment duration were also significant for these lipids, except for MGDG (P < 0.05) (Supplementary Table S1). The lipid species that exhibited the highest treatment-specific responses were all of high abundance in each lipid class. MGDG showed a dose- and time-dependent decrease of 1.01- to 1.1-fold in the treated thalli due to a 1.1- to 1.6-fold decrease in the contents of C36 (36:4, 36:5 and 36:6), C38 (38:5 and 38:4) and C40 (40:8) lipid molecular species (Supplementary Fig. S2). PG content decreased with an increase in the MeJA dose in the treated thalli (1.1- to 1.6-fold) due to a decrease in C34 (34:3, 34:2 and 34:1), C36 (36:4 and 36:2), C37 (37:2 and 37:1) and C40 (40:8, 40:7) lipid molecular species (Supplementary Fig S3). However, an increase in the PG content (1.1- to 1.7-fold) was observed with time, except in 100 µM MeJA-treated thalli that had a lower PG content at 24 h. The contents of the phospholipids PC, PE and PA increased by 1.03- to 1.4-fold with an increased MeJA dose (except 100 µM MeJA-treated thalli). However, the response of the phospholipids PC, PE and PA was not uniform for each MeJA treatment (1, 10 and 100 µM) and varied with time. For instance, the content of PC increased from 6 h to 12 h (1.4- to 1.7-fold), and then decreased (1.4- to 1.5-fold) at 24 h in thalli treated with 1 and 10 µM MeJA, while the thalli treated with 100 µM showed an increase in PC content up to 24 h. Similarly, for PE and PA content, there was no uniform trend (Fig. 4). There was a decrease in lipid species containing two 18:3 acyl carbons in both MGDG and DGDG (especially at longer durations), indicating that these lipids would have been hydrolyzed to produce 18:3. No such reduction in 18:3-containing phospholipids was observed, except for PA. Among the lysolipids, the contents of lyso-phosphatidylethanolamine (LPE) and lyso-phosphatidylcholine (LPC) increased in the treated thalli with an increase in the MeJA dose (1.1- to 2.1-fold, and 1.2- to 7.9-fold, respectively) and time (3.8- to 5.4-fold, and 1.4- to 1.5-fold, respectively), except for 100 µM MeJA-treated thalli, while lyso-phosphatidylglycerol (LPG) showed non-significant changes as compared with the control (Supplementary Fig. S6).
The FA profiles of treated and control thalli showed non-significant changes for almost all the FAs quantified except C16:0, C16:1 (n-9), C20:3 (n-6), C20:4 (n-6) and total saturated FAs (Supplementary Tables S1, S3). Although polyunsaturated fatty acids (PUFAs) did not show any significant changes, C18:2 (n-6) and C20:4 (n-6) showed 1.1- to 1.6-fold increases, respectively, in the treated thalli, except at 12 h, while C20:4 (n-6) showed a 1.2- to 1.4-fold increase in 1 and 100 µM MeJA-treated thalli with time. The non-significant changes in the FA profile indicated continued cycling of FAs, desaturation of saturated FAs/monounsaturated FAs (MUFAs) to PUFAs and their incorporation into different lipid molecular species.
Oxylipin contents and lipoxygenase activity
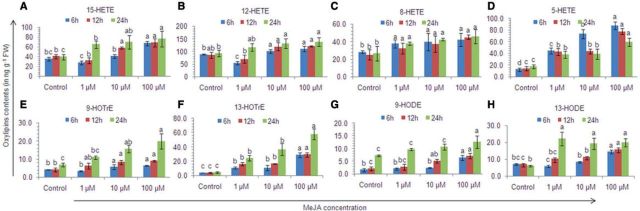
The oxylipin content significantly increased with the exogenous application of MeJA in both a dose- and time-dependent manner, except for 8-hydroxy-5,9,11,14-eicosatetraenoic acid (8-HETE) and 5-hydroxy-6,8,11,14-eicosatetraenoic acid (5-HETE) (P < 0.05) (Table 1; Fig. 5). The total oxylipin content increased by 1.03- to 2.1-fold, total hydroxyeicosatetraenoic acids (THETEs) by 1.1- to 1.9-fold, hydroxyoctadecadienoic acids (HODEs) by 1.3- to 2.5-fold and hydroxyoctadecatrienoic acids (HOTrEs) by 1.7- to 6.7-fold with an increase in the exogenous MeJA dose (except 1 µM MeJA-treated thalli). The increase in HOTrEs, especially 13-HOTrE (2.6- to 13.5-fold) showed the up-regulation of 13-LOX metabolism, as 13-hydroxy-9,11,5-octadecatrienoic acid (13-HOTrE) is produced by the reduction of a 13-LOX product, 13-hydroperoxy-9,11,15-octadecatrienoic acid (by peroxidases), one of the key substrates of JA biosynthesis. Similarly, the contents of 9-hydroxy-10,12-octadecadienoic acid (9-HODE) and 13-hydroxy-9,11-octadecadienoic acid (13-HODE) also increased in a dose-dependent manner (1.2- to 4.0-fold and 1.2- to 3.3-fold, respectively) and with time (1.4- to 4.8-fold and 1.1- to 3.8-fold, respectively). Among HETEs, the maximum increase was found in 5-HETE (2.2- to 6.9-fold) followed by 15-hydroxy-5,8,11,13-eicosatetraenoic acid (15-HETE) (1.1- to 1.9-fold) and 8-HETE (1.3- to 1.8-fold).
Table 1.
Oxylipin group contents (ng g–1 FW) in methyl jasmonate-treated Gracilaria dura (means ± SD, n = 3)
| Control | 1 µM MeJA | 10 µM MeJA | 100 µM MeJA | |
|---|---|---|---|---|
| Total oxylipin contents | ||||
| 6 h | 187.6 ± 9.6c | 192.8 ± 26.4c | 297.2 ± 36.2b | 380.7 ± 34.0a |
| 12 h | 191.1 ± 8.2c | 228.2 ± 16.2c | 302.9 ± 10.5b | 396.3 ± 28.5a |
| 24 h | 206.8 ± 6.2d | 328.8 ± 14.8c | 372.1 ± 32.3b | 430.4 ± 22.8a |
| THETE | ||||
| 6 h | 165.2 ± 10.2c | 164.6 ± 21.2c | 255.9 ± 30.5b | 308.2 ± 25.8a |
| 12 h | 166.0 ± 15.1b | 177.0 ± 26.4b | 259.5 ± 12.3a | 312.7 ± 24.1a |
| 24 h | 177.6 ± 7.4c | 259.0 ± 21.6b | 284.5 ± 28.0ab | 320.0 ± 13.4a |
| THOTrE | ||||
| 6 h | 8.4 ± 0.4c | 14.3 ± 1.7b | 16.5 ± 3.1b | 35.1 ± 3.0a |
| 12 h | 8.0 ± 2.4c | 22.5 ± 3.4b | 25.1 ± 1.3b | 38.4 ± 3.7a |
| 24 h | 11.2 ± 0.5d | 35.7 ± 4.3c | 52.3 ± 9.6b | 73.1 ± 9.4a |
| THODE | ||||
| 6 h | 8.6 ± 0.2c | 7.9 ± 0.9c | 10.8 ± 0.5b | 20.7 ± 2.1a |
| 12 h | 9.3 ± 0.8c | 13.0 ± 3.6bc | 16.5 ± 0.7b | 22.9 ± 2.6a |
| 24 h | 13.4 ± 0.7c | 32.0 ± 6.5b | 30.1 ± 5.1ab | 32.7 ± 4.6a |
MeJA, methyl jasmonate; THETE, total hydroxy-eicosatetraenoic acid; THOTrE, total hydroxy-octadecatrienoic acid; THODE, total hydroxy-octadecadienoic acid.
a-c: Values in a row are significantly different at P < 0.05.
Fig. 5.
Effect of methyl jasmonate on oxylipin contents in Gracilaria dura. (A) 15-HETE, (B) 12-HETE, (C) 8-HETE, (D) 5-HETE, (E) 9-HOTrE, (F) 13-HOTrE, (G) 9-HODE and (H) 13-HODE. Different letters on the same shaded columns indicate that mean values for the particular incubation time were significantly different at P < 0.05.
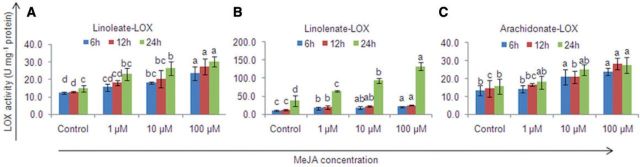
The LOX activities for the three substrate FAs [linoleic acid (LA), ALA and arachidonic acid (AA)] also increased significantly (P < 0.05) with an increase in the MeJA dose and with time, in agreement with an increase in the oxylipin content (Fig. 6). The maximum increase was shown by linolenate-LOX (ALA-LOX) (1.6- to 3.5-fold with an increase in the MeJA dose and 1.1-to 6.3-fold with time).
Fig. 6.
Effect of methyl jasmonate on lipoxygenase (LOX) activity in Gracilaria dura. (A) linoleate LOX, (B) linolenate LOX and (C) arachidonate LOX. Different letters on the same shaded columns indicate that mean values for the particular incubation time were significantly different at P < 0.05.
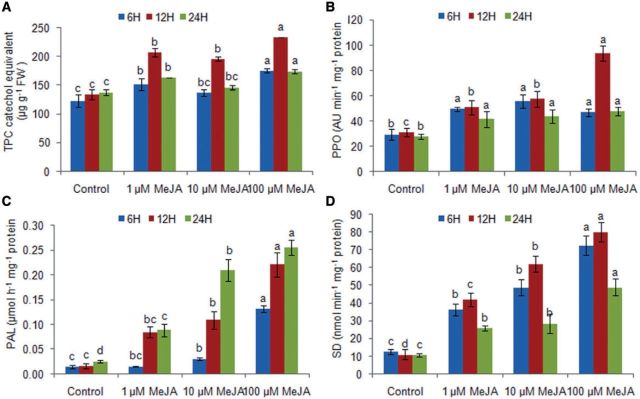
Total phenolic compounds (TPCs), polyphenol oxidase (PPO), phenylalanine ammonia-lyase (PAL) and shikimic dehydrogenase (SD) activities
MeJA-treated thalli showed a significant dose- and time-dependent increase in the content of TPCs (by 1.2- to 1.7-fold and 1.1- to 1.4-fold, respectively) as compared with the control (P < 0.05) (Fig. 7). However, the content of TPCs in the treated thalli was 1.3-fold lower at 24 h than at 12 h. The increase in TPCs was in agreement with an increase in the activity of PPO, which increased by 1.5- to 3.0-fold with an increase in the MeJA dose (Fig. 7). PAL activity increased significantly in the treated thalli with an increase in the MeJA dose (1.5- to 13.2-fold) as well as time (1.7- to 7.0-fold increase) as compared with the control (P < 0.05) (Fig. 7). Further, SD activity also increased by 2.4- to 7.2-fold in a dose-dependent manner in the treated thalli as compared with the control. However, SD activity increased up to 12 h with an increase in time (1.1- to 1.3-fold) and then decreased at 24 h.
Fig. 7.
Effect of methyl jasmonate on phenolic compounds and the activities of enzymes polyphenol oxidase (PPO), phenylalanine ammonia-lyase (PAL), and shikimate dehydrogenase (SD) in Gracilaria dura. Different letters on the same shaded columns indicate that mean values for the particular incubation time were significantly different at P < 0.05.
Discussion
In the present study, MeJA induced a state of oxidative stress in G. dura thalli due to ROS production (H2O2, HO· and O2·−) (Figs. 1, 2), followed by lipid peroxidation and induction of the downstream FA oxidation cascade leading to the production of hydroxylated oxylipins. MeJA is a stress hormone and a potent inducer of ROS production and oxidative stress (Collén et al. 2006, Küpper et al. 2009). This study showed a dose- and time-dependent increase in production of ROS, with the strongest response observed at 100 µM. In line with the present study, the effects of MeJA in red algae showed were maximal at 6 h after its application (Collén et al. 2006). However, the most recent report by Küpper et al. (2009) in brown algae reported earlier responses to the application of MeJA, measuring an oxidative burst that reached a maximum of accumulation of ROS concomitant with the liberation of free PUFAs and oxylipin formation during the first hour following the addition of the MeJA. This previous report did not attempt to characterize any other long-term responses except the establishment of resistance against the pathogenic endophyte L. tomentosoides. Furthermore, the enhanced ROS production as a function of MeJA treatment, especially at higher concentrations of 10 and 100 µM, has also been observed in microalgae (Fedina and Benderliev 2000, Kováčik et al. 2011) and higher plants (Jung 2004, Maksymiec and Krupa 2006). There could be multiple sources of ROS in this study such as NAD(P)H oxidase, a neutrophil-like superoxide-generating enzyme and peroxidases (Zambounis et al. 2012). This oxidative response could also be related to the reported changes in the photosynthetic apparatus that may lead to some disruption in the electron flow leading to ROS production. However, it requires further investigation of the effect of the MeJA on the photosynthetic parameters using PAM fluorometry. Collén et al. (2006) attributed the production of ROS to NADPH oxidase as apparent from its increased transcripts in MeJA-treated C. crispus, while Küpper et al. (2009) found that the source of ROS was only partially inhibited by diphenylene iodonium [a suicide substrate inhibitor of NAD(P)H oxidases]. Indeed, the accumulation of ROS in G. dura in the present study was statistically significant; instead it did not increase dramatically, possibly resulting from the balance between the production and consumption of ROS by antioxidative enzymes and thus the activation of early defensive systems against ROS. The antioxidative enzymes including periredoxin, catalase, ascorbate peroxidase, superoxide dismutase and methionine sulfoxide reductase are reported to be up-regulated at the transcriptional level in response to the MeJA in the red alga C. crispus, suggesting an involvement of active oxygen in MeJA signaling (Collén et al. 2006). This indicates that the MeJA signaling in red algae involves ROS production and the oxidative burst, and is modulated by increased expression of antioxidative enzymes. MeJA-induced oxidative stress plays a crucial role in conferring resistance against algal endophytes such as Acrochete operculta in C. crispus (Bouarab et al. 2004) and Laminariocolax tomentosoides in L. digitata (Küpper et al. 2009). A specific cross-talk has been observed between MeJA and ROS levels (H2O2) that act as secondary messengers and aid in up-regulation of various stress genes such as glutathione S-transferase, heat shock protein 20, xenobiotic reductase and genes involved in the phenylpropanoid pathway (Collén et al. 2006, Liu et al. 2008).
Increased ROS production due to different stress conditions results in lipid peroxidation in seaweeds including Gracilaria spp. (Kumar et al. 2010, Kumar et al. 2011), G. dura in the present study, E. siliculosus (Ritter et al. 2014) and L. digitata (Ritter et al. 2008). This further damaged the photosynthetic pigments, especially Chl a (Fig. 3). Similar decreases in pigments and cell viability have been observed in microalgae and freshwater algae treated with higher doses of MeJA (10–4–10–5 M or higher) (Fedina and Benderiev 2000, Czerpak et al. 2006, Piotrowska et al. 2010). In macroalgae, Gaquerel et al. (2007) reported that 100 µM MeJA was not detrimental to C. crispus thalli, and observed strong necrosis only at concentrations >100 µM. However, G. dura in the present study showed the symptoms of depigmentation and bleaching at the 100 µM MeJA dose at 48 h, possibly due to the higher levels of ROS accumulation. The degradation of Chl a has also been observed in higher plants in response to MeJA-induced oxidative stress (Ananieva et al. 2007, Gómez et al. 2010). A decrease in Chl a content was accompanied by an increase in phycobiliproteins (especially phycoerythrin), as found in Gracilaria thalli exposed to abiotic stresses (Kumar et al. 2010, Kumar et al. 2011). However, in the present study, there was a decrease in phycocyanin content at 24 h after an initial increase up to 12 h (Fig. 3), in congruence with the earlier report of increased transcripts of phycocyanin lyase found in MeJA-treated C. crispus (Collén et al. 2006). In contrast, allophycocyanin did not show any significant changes in G. dura in the present study. This indicated that phycocyanin is more vulnerable to oxidative damage among phycobiliproteins in G. dura.
MeJA at a lower concentration (1 µM) increased the total lipid content throughout the studied period, while MeJA at higher doses (of 10 and 100 µM) caused a decrease in the total lipid content over a longer incubation period (24 h). This is in agreement with the dose- and time-dependent increase in lipid peroxidation (Fedina and Benderiev 2000, Piotrowska et al. 2010). Recently, Jusoh et al. (2015) showed that the application of JA (45 µM) to the microalgal cell cultures of Chlorella vulgaris increased lipid accumulation by 54% and could be utilized in microalgal cultivation to facilitate commercial mass production of microalgal lipids. Furthermore, quantitative elecrospray ionization–mass spectroscopy (ESI-MS) profiling revealed that G. dura lipids were highly unsaturated and exhibited a large amount of long-chain PUFAs (C18:2, 20:3 and 20:4) in their polar lipids (Supplementary Table S2; Supplementary Figs. S2–S6). The lipid species containing C40 acyl chains contributed to 20–30% of total polar lipids and were mostly localized in MGDG, in agreement with earlier reports (Khotimchenko 2002). The presence of C20:4 and C20:5 in MGDGs revealed that these FAs were imported from the endoplasmic reticulum and thereafter incorporated into the chloroplastic galactolipids (Khotimchenko 2002). The analysis revealed that MGDG, PC, PE and PA showed significant dose- and time-dependent changes in response to MeJA treatment (Fig. 4). MGDG and PC were the most affected lipid classes, perhaps due to the high flux of these lipid classes during lipid metabolism as they are the primary sites for de novo FA allocation (Ohlrogge and Browse 1995). As the flux through these lipid classes is greater than through other classes, they are more sensitive to changes in precursor supply. Moreover, the lipid species with higher abundances exhibited the highest treatment-specific responses in each lipid class. There was a 1.1- to 1.6-fold decrease in MGDG in response to MeJA (1–100 µM), while the contents of PG decreased only at higher MeJA concentration (100 µM). DGDG contents were almost constant at 6 and 12 h, and showed a 1.1- and 1.2-fold increase in 1 and 10 µM-treated thalli, respectively, contrasting with a 1.1-fold decrease in 100 µM-treated thalli at 24 h. In contrast, MeJA did not alter the overall lipid content in Silybum marinum cells treated with 100 µM MeJA up to 48 h, except for a small progressive increase in DGDG and MGDG (Cacho et al. 2012). The contents of the phospholipids PC, PE and PA increased, except at the higher MeJA doses, while a decrease in PE and PA was observed in G. dura. The contents of the minor lipids PS and PI were not significantly altered. This indicated that most of the fatty acyl chains were degraded from MGDG and included C18:2, C18:3, C20:3 and C20:4 that were channeled downstream in the FA oxidation pathway leading to the production of oxylipins, as evident from higher LOX activities for LA, ALA and AA (Fig. 6). Lion et al. (2006) also showed that dihydroxylated FAs were released directly from galactolipids in Gracilaria chilensis in response to wounding. It is noteworthy that the decrease in lipid molecules containing 18:3 (36:6 and 36:5) was found mainly in the galactolipids MGDG and DGDG (which showed a decrease only at 24 h), while most of the phospholipids showed an increase in 36:6 acyl lipid chains, except PI in G. dura. This indicated that MGDG could play a crucial role in JA/MeJA biosynthesis (if it occurs in Gracilaria spp., as endogenous JA/MeJA has not been detected to date in Gracilaria spp.) and that the JA/MeJA-mediated defense responses are analogous to those reported in higher plants (Hyun et al. 2008, Wang 2009). Transgenic plants in which MGDG synthase activity was down-regulated by using RNA interference technology produced lower levels of JA than wild-type plants in response to wounding. Hyun et al. (2008) also reported that chloroplast lipid hydrolysis is a critical step for JA biosynthesis. The high levels of PA (40:8, 40:7, 38:5, 38:4, 36:4 and 36:3) and the lyso-lipids LPC (20:4, 20:3, 18:3 and 18:2) and LPE (20:4) probably generated from PC and PE in the treated thalli (Supplementary Fig. S6), indicating higher phospholipase activity and phospholipid turnover in G. dura, are consistent with the earlier reports of Profotova et al. (2005) and Cacho et al. (2012). Despite the significant changes in polar lipid composition of G. dura under MeJA response, the FA composition was not altered significantly except for C16:0, C16:1 (n-9), C20:3 (n-6) and C20:4 (n-6), similar to the earlier reports in brown macroalgae (Dictyota dichotoma, Colpomenia peregrina, Ectocarpus fasciculatus, Fucus vesiculosus, Himanthalia elongata and Saccharina latissima) and Silybum marianum (Wiesemeier et al. 2008, Küpper et al. 2009, Cacho et al. 2012). Moreover, no changes were found in brown macroalgal samples on elicitation with JA/MeJA at ecologically relevant conditions (0.1–0.5 mg ml–1), and significant metabolic changes were observed only at concentrations >0.5 mg ml–1, which was much higher than the concentrations applied in the present study. At such a higher exogenous application of JA, there was up-regulation of 16:0, 16:1 and 18:1 in all the brown macroalgae investigated (Wiesemeier et al. 2008).
Although, Gracilaria spp. contain both the C18 and C20 fatty acid oxidative pathways, the biosynthesis of JA/MeJA from C18 PUFAs via the C18 oxidative pathway is not defined, but the formation of hydroperoxy- and hydroxy-FAs from both the C18 and C20 PUFAs is well documented under normal and stress conditions (Lion et al. 2006, Weinberger et al. 2011, Rempt 2012). In addition, dihydroxylated eicosanoid, 7S,8R-dihydroxy eicosatetraenoic acid (7,8-di-HETE) is released along with free FAs and 8R-hydroxy eicosatetraenoic acid (8-HETE) upon wounding (Lion et al. 2006). These previous reports in Gracilaria spp. mostly emphasized the importance of AA(C20)-derived metabolites in response to wounding and other stresses, while the importance of C18-derived oxylipins was not so clear. Here, the significance of both the C18- and C20-derived oxylipins is deciphered in response to MeJA in G. dura. MeJA induced a cascade of oxygenation of C18 and C20 PUFAs (C18:2, C18:3 and C20:4), leading to a dose- and time-dependent accumulation of hydroxy-oxylipins (HETEs, HODEs and HOTrEs) (Fig. 5). A similar increase in hydroxy-oxylipins such as 13-HODE, 13-oxo-ODE, 15-HETE and 12-HETE, as well as ketols derived from C18:2 and C20:4 has been reported in C. crispus and L. digitata when treated with MeJA (Bouarab et al. 2004, Gaquerel et al. 2007, Küpper et al. 2009). This increase was more pronounced at higher concentrations (10 and 100 µM) as reported earlier (Bouarab et al. 2004, Gaquerel et al. 2007, Küpper et al. 2009). The higher content of 13/9-HOTrE (during the entire 24 h duration) and 13/9-HODE (at 24 h) indicated the up-regulation of the 13-LOX pathway. Similar increases in the activities of 5-LOX, 15-LOX and 8-LOX were observed, as apparent from higher increases in 5-HETE, 15-HETE and 8-HETE contents, respectively. Moreover, the accumulation of these hydroxy-oxylipins occurred concomitantly with the oxidative burst, as found in L. digitata (Küpper et al. 2009). These authors further illustrated that although it is not known whether the oxidation of PUFAs occurs after or before their release from membranes, the latter possibility is more pronounced as LOX isoforms that oxidizes PUFAs attached to lipids, such as MGDG, DGDG, PG and PC, are already reported in higher plants (Buseman et al. 2006, Vu et al. 2011). The untargeted profiling of oxidized lipids may help in gaining insight in this regard in macroalgae. Further, MeJA was found to be a potent enhancer of LOX (LA-, ALA- and AA-LOX) as inferred by an increase in HODEs, HOTrEs and HETEs in the treated thalli (Bouarab et al. 2004, Gaquerel et al. 2007, Küpper et al. 2009). In G. dura, the highest increase was observed in ALA-LOX followed by LA- and AA-LOX (Fig. 6). Similarly, LOX had relatively less specificity for arachidonate as compared with linoleate/linolenate in C. crispus and this provided the scope for bisallylic hydroxylation of PUFAs (Gaquerel et al. 2007).
MeJA also significantly increased the content of phenolic compounds and enzymes involved in secondary metabolism such as PPO, PAL and SD (Fig. 7), analogous to higher plants (Bouarab et al. 2004, Liu et al. 2008, Cacho et al. 2012, Gharechahi et al. 2013). A single brief exposure to airborne MeJA at low concentrations (5.42–542 nM) during the periods of tidal emergence significantly increased the polyphenolic contents (phlorotanins by 1.6-fold) in the brown alga F. vesiculosus (Arnold et al. 2001). The timing and magnitude of the induced increase in phlorotanin concentration was similar to those caused by real or simulated herbivory, indicating the putative role of MeJA in antiherbivore responses in algae. Recently, Chowdhury et al. (2014) also showed that the addition of 2 µM MeJA for 24 h to post-harvest cultures of Ecklonia cava increased the crude phlorotanin (brown algal polyphenol) content by 156%. In contrast, Collén et al. (2006) found that only the transcripts for DHAP (3-deoxy-d-arabino-heptulosonic acid-7-phosphate) synthase in a C. crispus microarray was overexpressed at 6 h, while no other transcripts involved in the shikimate pathway were identified. Further, the maximum accumulation of TPC was found at 12 h in G. dura, and then its content decreased at 24 h as compared with the control, concomitant with the decrease in PPO activity at 24 h. Similarly, SD activity also decreased at 24 h as compared with the control while maximum PAL activity was found at 24 h. This indicated that PAL activity lagged behind those of PPO and SD, or, conversely, PAL activity was induced in the late phase of MeJA-induced oxidative stress as compared with PPO/SD. Similarly, no significant change was observed in PAL activity in pea leaves during the initial 12–14 h of JA treatment, with the maximum activity observed at 36–48 h of JA application (Liu et al. 2008). Moreover, the induction of PAL activity in response to JA/MeJA has been directly linked to the H2O2 burst, and it was found that PAL activity can be completely blocked by pre-treatments with H2O2 scavengers (superoxide dismutase and catalase) and quenchers (Liu et al. 2008).
Thus, this study establishes the signaling role of MeJA in the red alga G. dura mediated by ROS-induced oxidative stress that leads to lipid peroxidation, followed by the induction of the FA oxidation cascade and the up-regulation of enzymes involved in secondary metabolism. Nevertheless, MeJA has not been identified in any macroalgae, and this study also does not provide any evidence of its presence in G. dura (authors’ personal observation). This enigma raises the question of the physiological role of this compound in macroalgae in general including Gracilaria spp. (in this study). The genome of the brown alga E. siliculosus does not contain homologs of JA receptors and components of the core transcriptional regulatory complex (MYCs, JAZs, NINZA and COI1) (Cock et al. 2010), indicating that MeJA may not have the same function in brown algae as in land plants or it has evolved to serve similar functions using different regulatory systems (Rittter et al. 2014). However, the presence of AOS and AOC genes involved in the initial step of JA biosynthesis in the Ectocarpus genome (Cock et al. 2010) indicates the role of putative cyclopentanones in brown algae. This is further supported by the accumulation of C18 cyclic oxylypins such as 12-OPDA, phytoprostanes (PPA1 and PPA2) and C20 cyclic prostaglandins (PGA2, PGB2, PGD, PGE, PGE2, 15-keto-PGE2α, 15-keto-PGA2 and PGJ2) in E. siliculosus and L. digitata under copper stress (Ritter et al. 2008, Ritter et al. 2014). OPDA and phytoprostanes have well defined roles in environmental stress and pathogenesis in higher plants, acting independently of MeJA (Stotz et al. 2013). Although, the red algal genome of C. crispus does not contain any AOS or AOC genes, it contains two candidate genes for 12-OPR and genes involved in β-oxidation within the JA biosynthetic pathway (Collén et al. 2013), suggesting that different sets of enzymes are involved in JA biosynthesis in red algae and/or they are regulated by an entirely different set of enzymes. Several studies have demonstrated the role of MeJA as a stress hormone in both brown and red algae, in defense against endophytic pathogens and grazers in antiherbivory (Arnold et al. 2001, Bouarab et al. 2004, Collén et al. 2006, Gaquerel et al. 2007, Küpper et al. 2009). In addition, recent studies have also implicated the role of JA/MeJA in promoting microalgal growth (51% increase in cell density in C. vulgaris cultures) and in considerably enhancing microalgal lipid and FA (C16:0, C18:0, C18:1 and C18:3) production in the stationary growth phase (Jusoh et al. 2015). The post-harvest MeJA treatment with 2 µM MeJA for 24 h was not only found to be useful for improving the yield of phlorotanin from E. cava but it also significantly enhanced the viability of E. cava tissues, indicating that this compound may prevent the post-harvest decay of algal tissues at least in the short term (Chowdhury et al. 2015). The present study and the above-mentioned observations support that MeJA has an active role in physiological responses in algae. There is a need for stronger efforts to improve knowledge of the pathways of oxylipin biosynthesis including JA using the combined omics approach of genomics, transcriptomics and metabolomics, and to study the role of this compound in different abiotic (salinity, desiccation, light and temperature) and biotic stresses (pathogens, grazers and herbivory) that can elucidate the direct relevance of this compound in macroalgae. In this context, the study of the role of MeJA in Gracilaria spp. in response to their pathogenic bacteria and fungi or simulated herbivory studies in the laboratory would be an ideal approach to confirm the putative physiological roles of MeJA in stress/defense in G. dura claimed in this study; at present it is beyond the scope of this study.
In conclusion, MeJA is a lipid-derived signaling compound that originates from the 13-LOX pathway of PUFA oxidation. Although the occurrence of MeJA in macroalgae is not clear, it is widely presumed that as higher plants evolved from algae, MeJA plays an analogous role in macroalgae to that in higher plants, where it regulates a plethora of developmental and stress responses. The present study revealed that MeJA (1–100 µM) was a strong elicitor of ROS production (H2O2, HO· and O2·−) in G. dura thalli and induced oxidative stress. This led to lipid peroxidation and degradation of Chl a and phycocyanin with concomitant increase in phycoerythrin. The MeJA-induced oxidative burst also induced the fatty acid oxidation cascade, resulting in the synthesis of hydroxy-oxylipins and up-regulation of 13-LOX. Most of these FAs were obtained from the degradation of the chloroplastic glycerolipid MGDG. In addition, G. dura thalli modulated the lipid acyl chains in such a way that no significant change was observed in the FA profile of the MeJA-treated thalli as compared with the control, except for C16:0, C16:1 (n-9), C20:3 (n-6) and C20:4 (n-6). This could be a strategy to maintain membrane integrity to counter oxidative stress. Furthermore, MeJA caused a shift towards secondary metabolism as a defense strategy in treated G. dura and caused the accumulation of phenolic compounds as well as the up-regulation of the enzymes involved in secondary metabolism, PPO, SD and PAL. It is clear from this study that MeJA induced an oxidative burst that significantly affected lipid metabolism and the FA oxidation cascade, and up-regulated secondary metabolism in G. dura.
Materials and Methods
Algal culture and MeJA treatment
Gracilaria dura was collected from the Adri coast (N 20°57.58′; E 70°16.76′), Gujarat, India in March 2010. The selected healthy thalli were carried in a cool pack to the laboratory and cleaned with autoclaved seawater to remove epiphytes. The cleaned algal thalli were maintained under laboratory conditions in aerated flat-bottomed round flasks in Provasoli enrichment seawater medium (Provasoli 1968) at 25 ± 1°C under daylight white fluorescent lamps at 15 µmol photon m–2 s–1 with a 12/12 h light/dark photoperiod. The culture medium was renewed weekly.
For MeJA treatment, healthy algal thalli maintained under laboratory conditions (in triplicate) were treated with increasing concentrations of MeJA (1, 10 and 100 µM) in ethanol in autoclaved seawater for variable time periods (6, 12, 24 and 48 h). In addition, untreated algal thalli were incubated with the same amount of ethanol for the solvent control for the same time periods. The detailed description of preparation of different concentrations of MeJA and solvent control is given in Supplementary protocol S1.
Determination of lipid peroxidation and ROS, and in situ localization of ROS
The level of lipid peroxidation was determined by TBARS resulting from the thiobarbituric acid reaction (Heath and Packer 1968). The superoxide (O2·−) production rate was determined according to the method published by Liu et al. (2010), H2O2 by recording the oxidation of aminoantipyrine at 510 nm, and the hydroxyl radical (HO·) production rate by following the method of Halliwell (2006). In situ localization of O2·− and H2O2 production was performed using NBT and DAB, respectively (Castro-Mercado et al. 2009). The detailed methodology is available in Supplementary protocol S1.
Pigment analyses
Chl a was extracted in 80% acetone, and phycobiliproteins in 100 mM phosphate buffer, pH 6.5 (1:4, w/v) in cool and dark conditions (Dawes et al. 1999). Phycocyanin, allophycocyanin and phycoerythrin contents were estimated using the equations as described by Tandeau and Houmard (1988). A detailed description of pigment analysis is available in Supplementary protocol S1.
Determination of total lipids, polar lipid profiling and fatty acids
Lipids were extracted by the modified Bligh and Dyer method (Kumari et al. 2011) using chloroform/methanol/phosphate buffer (pH 7.5) (1:2:0.9, by vol.). The lipid extracts were completely dried in glass vials and filled with nitrogen. The samples were sent to the Kansas State Lipidomics Research Center Analytical Laboratory, USA, for quantitative analysis of the polar lipid profile of MeJA-treated and control samples. FA analysis was accomplished by transmethylation of lipid samples (Carreau and Dubacq 1978). Fatty acid methyl esters (FAMEs) were extracted in hexane and separated on an RTX-5 fused silica capillary column, 30 m×0.25 mm×0.25 µm (Rastek) by gas chromatography–mass spectrometry (GC-MS) with helium (99.9% purity) as the carrier gas. GC-MS conditions were the same as in our earlier study (Kumari et al. 2011). A detailed description of ESI-MS lipidomic and FA analysis is provided in Supplementary protocol S1.
Determination of oxylipins and LOX activity
Algal samples were homogenized in ice-cold ethyl acetate containing 0.001% butylated hydroxytoluene under argon for 1 h at 4°C (Küpper et al. 2006, modified by Kumari et al. 2014). LOX enzyme was extracted according to the modified Tsai et al. (2008) method by monitoring the increase in absorbance at 234 nm with 100 µM substrate solutions of LA, ALA acid and AA prepared in ethanol. A detailed description is provided in Supplementary protocol S1.
Determination of total phenolic compounds, polyphenol oxidase, phenylalanine ammonia-lyase and shikimic dehydrogenase activities
TPCs in the algal samples were determined by following the method of Folin and Ciocalteu (1927), PPO by that of Chen et al. (2000), PAL by that of Qin and Tian (2005), and SD by that of Magalhães et al. (2002). A detailed description is provided in Supplementary protocol S1.
Statistical analysis
One-way ANOVA was carried out to compare the means between treatments with different concentrations of MeJA at P < 0.05. Two-way ANOVA was also performed with concentration and time as the two factors for all the parameters to identify the concentration- and time-dependent distinct responses to MeJA in G. dura.
Supplementary data
Supplementary data are available at PCP online.
Funding
This study was supported by the Council of Scientific and Industrial Research (CSIR) [senior research fellowship (SRF) CSIR Award No. 31/028(0101)/2009-EMR-1> to P.K.]. Instrument acquisition and method development for lipid analysis at Kansas State Lipidomics Research Centre Analytical Laboratory were supported by the National Science Foundation [EPS 0236913>, MCB 0455318> and 0920663>, DBI 0521587>]; Kansas Technology Enterprise Corporation; K-IDeA Networks of Biomedical Research Excellence (INBRE) of the National Institute of Health [P20RR16475>]; Kansas State University.
Supplementary Material
Acknowledgements
We would like to thank the Kansas State Lipidomics Research Center (KLRC) for polar lipid analysis, and the Head, Analytical Sciences, CSIR-CSMCRI for permitting us to use the GC facility. We would also like to thank Profesor Jeff Leblond, Middle Tennessee State University, USA, and Professor Wendy Stirk, Research Centre for Plant Growth and Development, University of KwaZulu-Natal Pietermartizburg, South Africa for editing the manuscript for English language as well as for their valuable comments which helped in the improvement of the revised manuscript.
Glossary
Abbreviations
- AA
arachidonic acid
- AA-LOX
arachidonate lipoxygenase
- ALA
α-linolenic acid
- ALA-LOX
linolenate lipoxygenase
- ANOVA
analysis of variance
- AOC
allene oxide cyclase
- AOS
allene oxide synthase
- DAB
3,3′-diaminobenzidine
- DGDG
digalactosyldiacylglycerol
- ESI-MS
electrospray ionization-mass spectrometry
- FA
fatty acid
- GC-MS
gas chromatography–mass spectrometry
- HETE
hydroxyeicosatetraenoic acid
- 12-HETE
12-hydroxy-5,8,10,14-eicosatetraenoic acid
- 15-HETE
15-hydroxy-5,8,11,13-eicosatetraenoic acid
- 5-HETE
5-hydroxy-6,8,11,14-eicosatetraenoic acid
- 8-HETE-8-hydroxy-5
9,11,14-eicosatetraenoic acid
- H2O2
hydrogen peroxide
- HODE
hydroxyyoctadecadienoic acid
- 9-HODE
9-hydroxy-10, 12-octadecadienoic acid
- 13-HODE
13-hydroxy-9,11-octadecadienoic acid
- HOTrE
hydroxyoctadecatrienoic acid
- 9-HOTrE
9-hydroxy-10,12,15-octadecatrienoic acid, 13-HOTrE, 13-hydroxy-9,11,15-octadecatrienoic acid
- HpODE
hydroperoxyoctadecadienoic acid
- 9-HpODE
9-hydroperoxy-10,12-octadecadienoic acid
- 13-HpODE
13-hydroperoxy-9,11-octadecadienoic acid
- JA
jasmonic acid
- LA
linoleic acid
- LA-LOX
linoleate lipoxygenase
- LPC
lyso-phosphatidylcholine
- LPE
lyso-phosphatidylethanolamine
- LPG
lyso-phosphatidylglycerol
- LOX
lipoxygenase, MDA, malondialdehyde
- MeJA
methyl jasmonate
- MGDG
monogalactosyldiacylglycerol
- NBT
nitroblue tetrazolium
- 12-OPDA
12-oxo-phytodienoic acid
- 12-OPR
12-oxo-phytodienoic acid reductase
- PA
phosphatidic acid
- PAL
phenylalanine ammonia-lyase
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PPO
polyphenol oxidase
- PS
phosphatidylserine
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SD
shikimate dehydrogenase
- TBARS
thiobarbituric acid-reactive substances
- TPCs
total phenolic compounds
Disclosures
The authors have no conflicts of interest to declare.
References
- Ananieva K., Ananiev E.D., Mishev K., Georgieva K., Malbeck J., Kaminek M., et al. (2007) Methyl jasmonate is a more effective senescence-promoting factor in Cucurbita pepo (zucchini) cotyledons when compared with darkness at the early stage of senescence. J. Plant Physiol. 164: 1179–1187. [DOI] [PubMed] [Google Scholar]
- Arnold T.M., Targett N.M., Tanner C.E., Hatch W.I. (2001) Evidence for methyl jasmonate induced phlorotannin production in Fucus vesiculosus (Phaeophyceae). J. Phycol. 37: 1026–1029. [Google Scholar]
- Bouarab K., Adas F., Gaquerel E., Kloareg B., Salaün J., Potin P. (2004) The innate immunity of a marine red alga involves oxylipins from both the eicosanoid and octadecanoid pathways. Plant Physiol. 135: 1838–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. (2009) Jasmonate passes muster, a receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 60: 183–205. [DOI] [PubMed] [Google Scholar]
- Buseman C.M., Tamura P., Sparks A.A., Baughman E.J., Maatta S., Zhao J., et al. (2006) Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves. Plant Physiol. 142: 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacho M., Peláez R., Corchete P. (2011) Lipid composition of Silybum marianum cell cultures treated with methyl jasmonate. Biol. Plant. 56: 221–26. [Google Scholar]
- Carreau J.P., Dubacq J.P. (1978) Adaptation of macroscale method to the microscale for fatty acid methyl transesterification of biological lipid extracts. J. Chromatogr. 151: 384–390. [Google Scholar]
- Castro-Mercado E., Martinez-Diaz Y., Roman-Tehandon N., Garcia-Pineda E. (2009) Biochemical analysis of reactive oxygen species production and antioxidative responses in unripe avocado (Persea americana Mill var Hass) fruits in response to wounding. Protoplasma 235: 67–76. [DOI] [PubMed] [Google Scholar]
- Chen C., Bélanger R., Benhamou N., Paulitz T.C. (2000) Defense enzymes induced in cucumber roots by treatment with plant growth promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol. Mol. Plant Pathol. 56: 13–23. [Google Scholar]
- Chowdhury M.T.H., Cho J.-Y., Ahn D.-H., Hong Y.-K. (2014) Methyl jasmonate enhances phlorotannin production in the brown seaweed Ecklonia cava J. Appl. Phycol. 27: 1651–1656. [Google Scholar]
- Cock J.M., Sterck L., Rouzé P., Scornet D., Allen A.E., et al. (2010) The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465: 617–621. [DOI] [PubMed] [Google Scholar]
- Collén J., Hervé C., Guisle-Marsollier I., Léger J.L., Boyen C. (2007) Expression profiling of Chondrus crispus (Rhodophyta) after exposure to methyl jasmonate. J. Exp. Bot. 57: 3869–3881. [DOI] [PubMed] [Google Scholar]
- Collén J., Porcel B., Carré W., Ball S.G., Chaparro C., et al. (2013) Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA 110: 5247–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerpak R., Piotrowska A., Szulecka K. (2006) Jasmonic acid affects changes in the growth and some components content in alga Chlorella vulgaris. Acta Physiol. Plant. 28: 195–203. [Google Scholar]
- Dawes C.J., Orduna-Rojas J., Robledo D. (1999) Response of the tropical red seaweed Gracilaria cornea to temperature, salinity and irradiance. J. Appl. Phycol. 10: 419–425. [Google Scholar]
- Fedina I.S., Benderliev K.M. (2000) Response of Scendesmus incrassatulus to salt stress as affected by methyl jasmonate. Biol. Plant. 43: 625–627. [Google Scholar]
- Folin O., Ciocalteu V. (1927) On tyrosine and tryptophan determinations in protein. J. Biol. Chem. 12: 239–243. [Google Scholar]
- Fujii S., Yamamoto R., Miyamoto K., Ueda J. (1997) Occurrence of jasmonic acid in Dunaliella (Dunalielales Chlorophyta). Phycol. Res. 45: 223–226. [Google Scholar]
- Gaquerel E., Hervé C., Labrière C., Boyen C., Potin P., Salaün J. (2007) Evidence for oxylipin synthesis and induction of a new polyunsaturated fatty acid hydroxylase activity in Chondrus crispus in response to methyl jasmonate. Biochim. Biophys. Acta 1771: 565–575. [DOI] [PubMed] [Google Scholar]
- Gharechahi J., Khalili M., Hasanloo T., Salekdeh G.H. (2013) An integrated proteomic approach to decipher the effect of methyl jasmonate elicitation on the proteome of Silybum marianum L. hairy roots. Plant Physiol. Biochem. 70: 115–122. [DOI] [PubMed] [Google Scholar]
- Gómez S., Ferrieri R.A., Schueller M., Orians C.M. (2010) Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato. New Phytol. 188: 835–844. [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2006) Reactive species and antioxidants—redox biology is a fundamental theme of aerobic life. Plant Physiol. 141: 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M. (1992) Metabolism of 6,9,12-octadecatrienoic acid in the red alga Lithothamnion coralliodes: mechanism of formation of a conjugated tetraene fatty acid. Biochem. Biophys. Res. Commun. 188: 1220–1227. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Gardner H.W. (1992) Oxylipin pathway to jasmonates: biochemistry and biological significance. Biochim. Biophys. Acta 1165: 1–18. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Gerwick W.H. (1993) Biosynthesis of vicinal dihydroxy fatty acids in the red alga Gracilariopsis lemaneiformis: identification of a sodium-dependent 12-lipoxygenase and a hydroperoxide isomerase. Arch. Biochem. Biophys. 305: 115–122. [DOI] [PubMed] [Google Scholar]
- Heath R.L., Packer L. (1968) Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125: 189–198. [DOI] [PubMed] [Google Scholar]
- Hyun Y., Choi S., Hwang H., Yu J., Nam S., Ko J., et al. (2008) Cooperation and functional diversification of two closely related galactolipase genes for jasmonate biosynthesis. Dev. Cell 14: 183–192. [DOI] [PubMed] [Google Scholar]
- Jung S. (2004) Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol. Biochem. 42: 225–231. [DOI] [PubMed] [Google Scholar]
- Jusoh M., Loh S.H., Chuah T.S., Aziz A., Cha T.S. (2015) Elucidating the role of jasmonic acid in oil accumulation, fatty acid composition and gene expression in Chlorella vulgaris (Trebouxiophyceae) during early stationary growth phase. Algal Res. 9: 14–20. [Google Scholar]
- Khotimchenko S.V. (2002) Distribution of glyceroglycolipids in marine algae and grasses. Chem. Nat. Compd. 38: 223–229. [Google Scholar]
- Kombrink E. (2012) Chemical and genetic exploration of jasmonate biosynthesis and signaling paths. Planta 236: 1351–1366. [DOI] [PubMed] [Google Scholar]
- Kováčik J., Klejdus B., Štork F., Hedbavny J., Bačkor M. (2011) Comparison of methyl jasmonate and cadmium effect on selected physiological parameters in Scenedesmus quadricauda (Chlorophyta, Chlorophyceae). J. Phycol. 47: 1044–1049. [DOI] [PubMed] [Google Scholar]
- Krupina M.V., Dathe W. (1991) Occurrence of jasmonic acid in the red alga Gelidium latifolium. Z. Naturforsch. C 46: 1127–1129. [Google Scholar]
- Kumar M., Gupta V., Trivedi N., Kumari P., Bijo A.J., Reddy C.R.K., et al. (2011) Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta). Environ. Exp. Bot. 72: 194–201. [Google Scholar]
- Kumar M., Kumari P., Gupta V., Reddy C.R.K., Jha B. (2010) Biochemical responses of red alga Gracilaria corticata (Gracilariales, Rhodophyta) to salinity induced oxidative stress. J. Exp. Mar. Biol. Ecol. 391: 27–34. [Google Scholar]
- Kumari P., Reddy C.R.K., Jha B. (2011) Comparative evaluation and selection of a method for lipid and fatty acid extraction from macroalgae. Anal. Biochem. 415: 134–144. [DOI] [PubMed] [Google Scholar]
- Kumari P., Reddy C.R.K., Jha B. (2014) Quantification of select endogenous hydroxy-oxylipins from tropical marine macroalgae. Mar. Biotechnol. 16: 74–87. [DOI] [PubMed] [Google Scholar]
- Küpper F.C., Gaquerel E., Boneberg E., Morath S., Salaün J., Potin P. (2006) Early events in the perception of lipopolysaccharides in the brown alga Laminaria digitata include an oxidative burst and activation of fatty acid oxidation cascades. J. Exp. Bot. 57: 1991–1999. [DOI] [PubMed] [Google Scholar]
- Küpper F.C., Gaquerel E., Cosse A., Adas F., Peters A.F., Müller D.G., et al. (2009) Free fatty acids and methyl jasmonate trigger defense reactions in Laminaria digitata. Plant Cell Physiol. 50: 789–800. [DOI] [PubMed] [Google Scholar]
- Lion U., Wiesemeier T., Weinberger F., Beltrμn J., Flores V., Faugeron S., et al. (2006) Phospholipases and galactolipases trigger oxylipin-mediated wound-activated defence in the red alga Gracilaria chilensis against epiphytes. ChemBioChem 7: 457–462. [DOI] [PubMed] [Google Scholar]
- Liu Y., Jiang H., Zhao Z., An L. (2010) Nitric oxide synthase like activity-dependent nitric oxide production protects against chilling-induced oxidative damage in Chorispora bungeana suspension cultured cells. Plant Physiol. Biochem. 48: 936–944. [DOI] [PubMed] [Google Scholar]
- Liu Y., Pan Q.H., Yang H.R., Liu Y.Y., Huang W.D. (2008) Relationship between H2O2 and jasmonic acid in pea leaf wounding response. Russ. J. Plant Physiol. 55: 765–775. [Google Scholar]
- Magalhães M.L.B., Pereira C.P., Basso L.A., Santos D.S. (2002) Cloning and expression of functional shikimate dehydrogenase (EC 1.1.1.25) from Mycobacterium tuberculosis H37Rv. Protein Express. Purif. 26: 59–64. [DOI] [PubMed] [Google Scholar]
- Maksymiec W., Krupa Z. (2006) The effects of short-term exposition to Cd, excess Cu ions and jasmonate on oxidative stress spearing in Arabidopsis thaliana. Environ. Exp. Bot. 57: 187–194. [Google Scholar]
- Ohlrogge J., Browse J. (1995) Lipid biosynthesis. Plant Cell 7: 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska A., Bajguz A., Czerpak R., Kot K. (2010) Changes in the growth, chemical composition, and antioxidant activity in the aquatic plant Wolffia arrhiza (L.) Wimm. (Lemnaceae) exposed to jasmonic acid. J. Plant Growth Regul. 29: 53–62. [Google Scholar]
- Profotova B., Burketova L., Novotna Z., Martinec J., Valentova O. (2005) Involvement of phospholipases C and D in early responses to SAR and ISR inducers in Brassica napus plants. Plant Physiol. Biochem. 44: 143–151. [DOI] [PubMed] [Google Scholar]
- Provasoli L. (1968) Media and prospects for the cultivation of marine algae. In Cultures and Collection of Algae. Edited by Watanabe A., Hattori A. pp. 63–67. Japanese Society of Plant Physiologists, Tokyo. [Google Scholar]
- Qin G.Z., Tian S.P. (2005) Enhancement of biocontrol activity of Cryptococcus laurentii by silicon and the possible mechanisms involved. Biol. Control 95: 69–75. [DOI] [PubMed] [Google Scholar]
- Rempt M., Weinberger F., Grosser K., Pohnert G. (2012) Conserved and species-specific oxylipin pathways in the wound-activated chemical defense of the noninvasive red alga Gracilaria chilensis and the invasive Gracilaria vermiculophylla. Beilstein J. Org. Chem. 8: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter A., Dittami S.M., Goulitquer S., Correa J.A., Boyen C., Potin P., et al. (2014) Transcriptomic and metabolomic analysis of copper stress acclimation in Ectocarpus siliculosus highlights signaling and tolerance mechanisms in brown algae. BMC Plant Biol. 14: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter A., Goulitquer S., Salaün J., Tonon T., Correa J.A., Potin P. (2008) Copper stress induces biosynthesis of octadecanoid and eicosanoid oxygenated derivatives in the brown algal kelp Laminaria digitata. New Phytol. 180: 809–821. [DOI] [PubMed] [Google Scholar]
- Seo H.S., Song J.T., Cheong J.J., Lee Y.H., Lee Y.W., Hwang I. (2001) Jasmonic acid carboxyl methyltransferase, a key enzyme for jasmonate-regulated plant responses. Proc. Natl. Acad. Sci. USA 98: 4788–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz H.U., Mueller S., Zoeller M., Mueller M.J., Berger S. (2013) TGA transcription factors and jasmonate-independent COI1 signalling regulate specific plant responses to reactive oxylipins. J. Exp. Bot. 64: 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau N., Houmard J. (1988) Complementary chromatic adaptation. Physiological conditions and action spectra. Methods Enzymol. 167: 318–328. [Google Scholar]
- Tsai C.J., Li W.F., Pan B.S. (2008) Characterization and immobilization of marine algal 11-lipoxygenase from Ulva lactuca. J. Amer. Oil Chem. Soc. 85: 731–737. [Google Scholar]
- Ueda J., Miyamoto K., Aoki M., Hirata T., Sato T., Momotani Y. (1991) Identification of jasmonic acid from Euglena gracilis as a plant growth regulator. Bull. Osaka Prefect. Univ. Ser. B. Agric. Life Sci. 43: 103–108. [Google Scholar]
- Vu H.S., Tamura P., Galeva N.A., Chaturvedi R., Roth M.R., Williams T.D., et al. (2011) Direct infusion mass spectrometry of oxylipin-containing Arabidopsis membrane lipids reveals varied patterns in different stress responses. Plant Physiol. 158: 324–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. (2009) Monogalactosyldiacylglycerol deficiency affects jasmonic acid biosynthesis and defense responses to insect herbivores in Nicotiana tobacum. Plant Sci. 176: 279–285. [Google Scholar]
- Wasternack C., Forner S., Strnad M., Hause B. (2012) Jasmonates in flower and seed development. Biochimie 95: 79–85. [DOI] [PubMed] [Google Scholar]
- Weinberger F., Lion U., Delage L., Kloareg B., Potin P., Beltrán J., et al. (2011) Up-regulation of lipoxygenase, phospholipase, and oxylipin-production in the induced chemical defense of the red alga Gracilaria chilensis against epiphytes. J. Chem. Ecol. 37: 677–686. [DOI] [PubMed] [Google Scholar]
- Wiesemeier T., Jahn K., Pohnert G. (2008) No evidence for the induction of brown algal chemical defense by the phytohormones jasmonic acid and methyl jasmonate. J. Chem. Ecol. 34: 1523–1531. [DOI] [PubMed] [Google Scholar]
- Zambounis A., Gaquerel E., Strittmatter M., Salaün J., Potin P., Küpper F.C. (2012) Prostaglandin A2 triggers a strong oxidative burst in Laminaria: a novel defense inducer in brown algae? Algae 27: 21–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.