Abstract
Patients with neuropathic pain commonly present with spontaneous pain, in addition to allodynia and hyperalgesia. While evoked responses in neuropathic pain models are well characterized, determining the presence of spontaneous pain is more challenging. We determined if the chronic constriction injury (CCI) model could be used to measure effects of treatment of spontaneous pain, by evaluating dorsal horn neuron (DHN) spontaneous activity and spontaneous pain-related behaviors. We measured conditioned place preference (CPP) to analgesia produced by sciatic nerve block with bupivacaine in rats with established CCI. We undertook another CPP experiment using hindpaw incision. We also examined DHN spontaneous activity in CCI rats. While CCI produced nocifensive responses to mechanical stimuli, CPP to analgesic nerve block was not evident 14 days following injury: Compared to baseline (314 ± 65 sec), CCI rats did not show a preference for the bupivacaine-paired chamber following conditioning (330 ± 102 sec). On the other hand, sciatic nerve block after hindpaw incision produced CPP on postoperative day 1, serving as a positive control. The proportion of spontaneously active DHNs (33%) was not significantly increased in CCI rats compared to the sham (21%). The median rate of spontaneous activity in the CCI group (12.6 imp/s) was not different from the sham group (9.2 imp/s). Also, there was no change in DHN spontaneous activity following sciatic nerve block with bupivacaine. Our findings suggest that CCI as a neuropathic pain model should not be used to measure effects of treatment of spontaneous pain driven by the peripheral input.
Keywords: chronic constriction injury, spontaneous pain, dorsal horn neuron, spontaneous activity, conditioned place preference
Introduction
Spontaneous pain is an important aspect of most acute and chronic pain conditions. Initial signs of an effective treatment include evaluation against ongoing or spontaneous pain followed by evaluations of other parameters such as global improvement in pain, total pain relief, or increased activities tolerated. Spontaneous pain is a prominent feature of neuropathic pain conditions such as diabetic neuropathy, postherpetic neuralgia and trigeminal neuralgia [4; 5; 13; 27].
While many neuropathic pain patients present with symptoms of spontaneous pain, until recently we have been limited in measuring its presence in animal models of neuropathy. Conditioned placed preference (CPP) is one such laboratory test that is thought to indicate the presence of spontaneous pain [15; 16]. An important reason to identify the presence of spontaneous pain in an animal model is so we can properly study its treatment in the hopes of improving clinical outcomes in patients.
Spontaneous activity in dorsal horn neurons has been studied extensively in the spinal nerve ligation model of nerve injury [8; 24; 28]. The extent of spontaneous activity is modest in this model. We are interested in determining if other neuropathic pain models might have a more prominent component of spontaneous activity and spontaneous pain-related behaviors than spinal nerve ligation. Chronic constriction injury (CCI) model was shown to produce some spontaneous pain-related behaviors [2; 3]. We hypothesized that CCI would produce evidence of spontaneous pain when tested against CPP. We also hypothesized that spontaneous activity in dorsal horn neurons would be evident after CCI.
We propose to test these hypotheses by measuring CPP to analgesia produced by sciatic nerve block in rats with established CCI. We also examined the magnitude and prevalence of dorsal horn neuron spontaneous activity in CCI rats. We tested the response of spontaneously active dorsal horn neurons to the same sciatic nerve block used to test for CPP. If such an association could be demonstrated, then CPP and the measurement of dorsal horn neuron spontaneous activity in CCI model could be used to study the treatment efficacy of therapeutic interventions for ongoing pain.
Methods
All procedures in this study were approved by The University of Iowa Animal Care and Use Committee, Iowa City, Iowa, and conformed to the NIH guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats (200–224 gm) were housed in groups of 2 in clear plastic cages (40 × 60 × 30 cm) on fresh bedding with free access to food and water. The environment was controlled with 12 hours of light and 12 hours of dark with a non-fluctuating temperature of 22 ± 2°C.
CCI Surgery
CCI of the sciatic nerve was performed as described previously [3]. Briefly, rats were placed under anesthesia with isoflurane. After dissection and exposure of the sciatic nerve at the mid-thigh level, 4 ligatures of chromic gut suture (Ethicon Endo-Surgery, Blue Ash, Ohio) were loosely ligated around the nerve with approximately 1 mm spacing between sutures. Sham animals had the left sciatic nerve exposed; however, the nerve was not ligated.
Proximal Sciatic Nerve Block
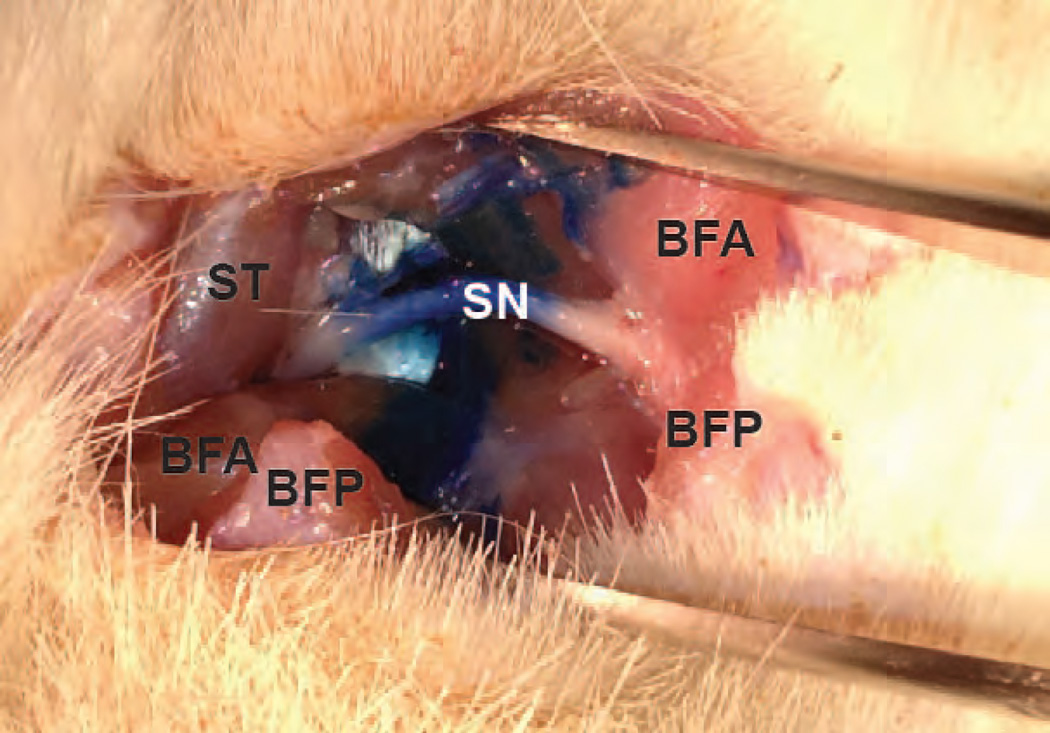
A proximal sciatic nerve block was used to block input from the CCI-induced injury under isoflurane anesthesia. This was performed using 200 µL of 0.5% bupivacaine (Hospira, Lake Forest, Illinois) or phosphate buffered saline (PBS, Invitrogen, Carlsbad, California), as a vehicle control. The nerve block was performed with the aid of a nerve stimulator using a 30-mm, 22-gauge nerve block needle (Smith-Medical, Keene, New Hampshire). A shaved region of the posterior thigh was palpated, and the groove created by the proximal portion of the hamstring muscles was identified. The stimulating needle was then inserted at this junction and aimed toward the groove created by the proximal femur and sacrum as described by Berde [17]. Stimulation frequency was set at 2 Hz and the pulse duration was set at 200 µsec. As the needle approached the sciatic nerve, dorsiflexion and/or plantar flexion of the ankle was observed. Prior to injection of the bupivacaine or vehicle, the stimulating needle was positioned to maximize the response at the ankle to a current of 0.5 to 0.55 mA. Using this combined landmark and stimulation technique for the sciatic nerve block, we were able to observe reversible loss of knee flexion, and ankle/hindpaw motor function in rats injected with bupivacaine. In one rat, we localized the sciatic nerve using this method, and then injected Methylene Blue dye (Thermo Fisher Scientific, Waltham, MA) to trace the distribution. In this rat, we observed that the injectate surrounded the proximal sciatic nerve as it coursed around the trochanter of the femur adjacent to the sacrum (Fig 1).
Fig. 1.
The distribution of Indigo Blue dye around the high sciatic nerve (SN), after a nerve stimulator-guided high sciatic nerve block. The proximal side is to the left and distal side to the right. The semitendinous (ST), anterior biceps femoris (BFA), and posterior biceps femoris (BFP) muscles are also labeled.
Plantar Incision
The plantar incision was made as previously described [6]. Briefly, the plantar aspect of the right hindpaw was prepared in a sterile manner, and a 1-cm longitudinal incision though skin, fascia and muscle was made. The skin was closed with 5-0 nylon sutures. Some rats underwent sham hindpaw incision. These animals underwent anesthesia and preparation of the hindpaw, but no incision was made.
Measurement of Pain Behaviors
Conditioned Placed Preference
For CPP experiments in CCI rats, all animals were handled by a single researcher (BD) and housed in a separate area from animals used for other experiments. Bedding and food pellets were changed by the same researcher. Animals were subjected to either CCI or sham surgery as described above. On POD 10–11, the animals underwent 5-min handling session each day. Following the first handling, the animals were placed in a fresh cage and transported to the animal care unit.
On preconditioning day, POD 12, baseline data were collected. Rats were placed in the CPP apparatus. The apparatus consisted of 3 chambers, two larger end chambers (46 × 32 × 32 cm) and one smaller center-connecting chamber (46 × 21.5 × 13 cm). The two end chambers had differing visual, olfactory and tactile cues. On the baseline measurement day, the animals were placed in the center with free access to all three chambers. The time spent in each chamber was recorded for 15 min to determine preconditioning baseline, and the preferred and non-preferred chambers were identified. Rats spending greater than 80% or less than 20% time in a chamber were excluded from further testing. Rats underwent single trial conditioning the following day POD13 [16; 23]. In the morning of the conditioning day, the animals received a high sciatic nerve administration of PBS and they were placed into the preferred chamber for 45 min, with no access to the other chambers. In the afternoon approximately 4 hours later, the rats received a high sciatic nerve block with drug treatment (0.5% bupivacaine or PBS) and were placed in the non-preferred chamber for 45 min, with no access to the other chambers. Thus, we used a biased approach [30]. On testing day, POD 14, the animals were placed back into the center of the CPP chambers with free access to all chambers for 15 min. The time spent in each chamber was again recorded. Three groups of animals were tested with 10 animals in each group. The first group was CCI rats, treated with PBS in the morning and bupivacaine in the afternoon (CCI-Bupivacaine group). The second group was CCI rats, treated with PBS in the morning and PBS in the afternoon (CCI-PBS group). The third group was sham-operated animals that received PBS in the morning and bupivacaine in the afternoon (Sham CCI-Bupivacaine group).
To verify that our CPP protocol could measure pain-related behavior, we undertook another CPP experiment using hindpaw incision. Preference for analgesia-paired chamber after hindpaw incision has previously been demonstrated [22]. As described for CCI animals, following the 2-day handling session by a single investigator, baseline preference for chambers was measured on preconditioning day. Exclusion criteria were the same as for CCI rats. Then, in the afternoon after taking baseline, rats underwent plantar hindpaw incision as described above. Rats underwent conditioning the following day, POD1. In the morning of the conditioning day, sciatic nerve administration of PBS was performed and rats were placed in the preferred chamber for 45 min. In the afternoon, sciatic nerve block with drug treatment (PBS or 0.5% bupivacaine) was performed and rats were placed in the non-preferred chamber for 45 min. The following day, POD 2, rats were placed in the center chamber and time spent in each chamber was measured for a total of 15 min. Three groups of animals were tested with 10 animals in each group. In the first group, rats underwent plantar incision, and then were treated with PBS in the morning and bupivacaine in the afternoon (Incision-Bupivacaine group). The second group underwent plantar incision, and was treated with PBS in the morning and the afternoon (Incision-PBS group). The third group was sham animals that received PBS in the morning and bupivacaine in the afternoon (Sham Incision-Bupivacaine group).
CPP data were expressed as either absolute measure of preference (comparison of total duration in the initially non-preferred chamber during preconditioning baseline vs. during the test period) or relative measure of preference (the Difference Score and the Time Preference Score). The Difference Score was calculated as “time (sec) spent in a chamber during the test period minus time spent in that chamber during preconditioning baseline.” When the animals were paired with bupivacaine in the non-preferred chamber on conditioning day, increased time spent (positive Difference Score) in the bupivacaine-paired chamber and decreased time spent (negative Difference Score) in the PBS-paired chamber would indicate the development of preference for the drug-paired chamber. In addition to Difference Score, the Time Preference Score was calculated by dividing the time spent in the initially non-preferred chamber during the test period by the time spent in both end chambers during the test period.
Nocifensive Response to Von Frey Filaments
To confirm the development of mechanical hypersensitivity following CCI, we determined the nocifensive response frequency elicited by three von Frey filaments. All animals were acclimated to a plastic mesh floor with a clear plastic cage top for 4–5 hours for 2 days prior to undergoing CCI or sham surgery. Following acclimation on the second day, CCI surgery was performed. The animals were then tested on postoperative day (POD) 2, 4, 7, 9, 11 and 14. Filament strengths of 58.82, 104.7 and 270.38 mN were tested. The filaments were applied to the middle of the hindpaw. Beginning with the weakest filament, each filament was applied 10 times with a minimum of 60 sec between applications. Withdrawal followed by nocifensive behaviors, either licking, lifting or flinching of the hindpaw, was considered a response [10]. Simple withdrawal without nocifensive behaviors was not considered a response. The percent responses was recorded [14].
Dorsal Horn Cell Recording
General
The study of the electrophysiology of dorsal horn neurons was performed on the animals 12–14 days following CCI surgery. The recording procedure was carried out in a similar fashion as previously described [25; 38]. Following induction of anesthesia, the rats were transported to the electrophysiology apparatus. They were initially maintained under anesthesia with 2% isoflurane with a nose cone. Temperature was measured with a rectal probe and maintained at 37 ± 2°C with an underbody heating pad, and a servo-controlled heating lamp. A carotid arterial line was placed for maintenance of mean arterial pressure of at least 90 mmHg. Animals were tracheotomized and mechanically ventilated (Harvard Apparatus, Inc, Millis, MA). The anesthetic depth was maintained at approximately 1.2% expired isoflurane and this depth prevented withdrawal to noxious tail pinch stimulation.
The paraspinal musculature was dissected from the spinous processes, laminae and transverse processes of the lower thoracic and upper lumbar vertebrae. The head was held in place with a stereotaxic holder, and the vertebral column were stabilized in metal clamps that were affixed to a rodent stereotaxic device (David Kopf Instruments, Tunjunga, CA). Limited laminectomies were performed to expose the dorsal spinal cord at the lumbar enlargement between 13th thoracic and 3rd lumbar vertebra. Small rat toothed forceps and the narrow-nosed Leksell rongeur were then used to widen the left spinal canal exposure. The dura was then opened with jewelers forceps and micro scissors. A few drops of mineral oil were placed over the exposed spinal cord to prevent drying. The pia was gently stripped from the surface of the dorsal columns of the lumbar enlargement of the spinal cord using jewelers forceps.
Recording of single unit action potentials was performed using Tungsten microelectrodes (1–1.2 MΩ Impedance; WPI, Sarasota, FL) attached to a hydraulic microdrive (David Kopf Instruments, Tunjunga, CA). Action potentials were amplified with a P5 series Grass amplifier (Grass Technologies, Warwick, RI), observed on an oscilloscope (Tektronix, Beaverton, OR), digitized with a 1401 Plus data acquisition unit, and recorded using Spike2 software (Cambridge Electronic Design, Cambridge, England). Only neurons with somatic receptive fields in the left hindleg and action potential amplitudes that were at least 2 to 3 times greater than action potentials of other neurons were recorded.
Recording Protocol
The electrode depth was set to 0 µm once the electrode entered the surface of the spinal cord. The electrode was then slowly advanced into the spinal cord at a rate of 10 µm/step using a hydraulic microdrive (David Kopf instruments, Tujunga, CA) until spontaneous neuronal activity was detected. If a neuron with SA was found, then innocuous mechanical search stimuli (tapping and touching) were applied throughout the hindleg to verify the neuron had a mechanical receptive field on the limb. Neurons were accepted for further study if their receptive fields included the hindpaw, gastrocnemius region or hamstring area up to the sacrum. If no SA was found until a maximal depth of approximately 1000 µm was reached, the electrode was then returned slowly (10 µm/step) to dorsal surface of the spinal cord, and at the same time, innocuous mechanical searching stimuli (tapping and touching) were applied to the hindlimb to identify any mechanosensitive neurons without SA.
Once a dorsal horn with a receptive field in the hindlimb was identified, the depth of the recording was noted, and the following protocol was employed. Before somatic stimulation, baseline activity of the neuron was recorded for 5 min, and the presence or absence of SA was determined: a neuron with a mean activity greater than 0.1 imp/sec over a 5 min period was considered spontaneously active. To further characterize the neurons, non-damaging pinch was applied using a small curved forceps to the hindpaw, gastrocnemius, and hamstring muscle, followed by flexion and extension of the ankle and knee joints, and then the brushing of the hindlimb (camel hair brush No.4). Each stimulus was approximately 3 sec duration, and the interstimulus interval was 5–10 sec unless there was continued activation. Any continued increase in action potential firing that lasted more than 2 sec following the cessation of a stimulus was considered positive for after discharge.
In some spontaneously active neurons, the effect of the high sciatic nerve block on the spontaneous activity was evaluated. The nerve block was usually performed in the afternoon after 2 or 3 other neurons had been recorded, First, the baseline SA was again recorded for 5 min. Then the high sciatic nerve block with 0.5% bupivacaine was performed as described for behavioral experiments. Fifteen min later, the SA was again recorded.
Neurons were classified based on the cutaneous classification scheme routinely used [25; 31; 37; 38]. In this study, both wide dynamic range (WDR) and high threshold (HT) neurons were included. The classification was based on response to brushing and to pinch. HT neurons were identified as neurons that only responded to pinch, but not to brush. WDR neurons responded to brush, touch and pinch as well, with greater responses to more intense stimuli. If any low threshold neurons responded maximally to brush but also had the same or smaller response to pinch, these cells were not studied. We recognize that these cells are studied 14 days after CCI, which could have converted a cell into a different somatic category. We refer to the neurons as WDR and HT.
Statistical Analysis
Kolmogorvo-Smirnov tests were applied to all continuous data sets to test for normality. Parametric tests were used for normally distributed, continuous data, which were expressed as mean ± standard deviation. Nonparametric analyses were used for non-normally distributed and categorical data. Non-normally distributed data were presented as median and interquartile range. Nocifensive response frequency to von Frey testing and the Time Preference Scores were analyzed using a two-way ANOVA with repeated measures on one factor followed by post-hoc Bonferroni test. The Difference Scores were compared by one-way ANOVA followed by post-hoc Tukey’s multiple comparison tests. Paired t-test was used to compare the absolute time spent by animals in the initially non-preferred chamber before and after conditioning, and the pre and post-block rate of SA. The proportions of spontaneously active dorsal horn neurons and the proportions of neurons with after discharge were compared between the two groups using a χ2-test. A nonparametric Mann-Whitney test was used to analyze the rate of SA between sham and CCI rats. Unpaired Student’s t-tests were used to compare the depth of recording. Results with values of P < 0.05 were considered statistically significant.
Results
Behavioral Studies
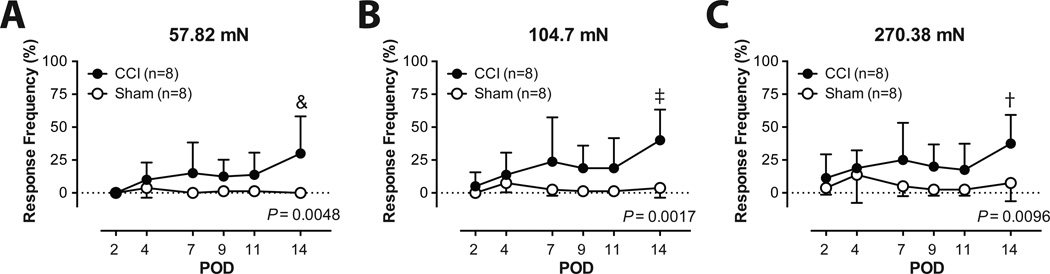
We confirmed the development of mechanical hypersensitivity following CCI. By POD 14, the mean nocifensive response frequency in the CCI group was 30 ± 28.3%, 40 ± 23.3% and 37.5 ± 21.9% respectively for the 58.82, 104.7 and 270.38 mN filament strengths. This compared to 0 ± 0% (P = 0.0048 vs. CCI), 3.75 ± 7.4% (P = 0.0017 vs. CCI) and 7.5 ± 13.9% (P = 0.0096 vs. CCI) in the sham group (Fig. 2).
Fig. 2.
Nocifensive response frequency to 57.82 mN (A), 104.7 mN (B), and 270.38 mN (C) von Frey filament application in the chronic constriction injury (CCI) and the sham groups. The results are expressed as mean and standard deviation. &P < 0.0001; ‡P < 0.001; and †P < 0.01 vs. Sham, two-way ANOVA with repeated measures on one factor, followed by post-hoc Bonferroni test. POD = postoperative day.
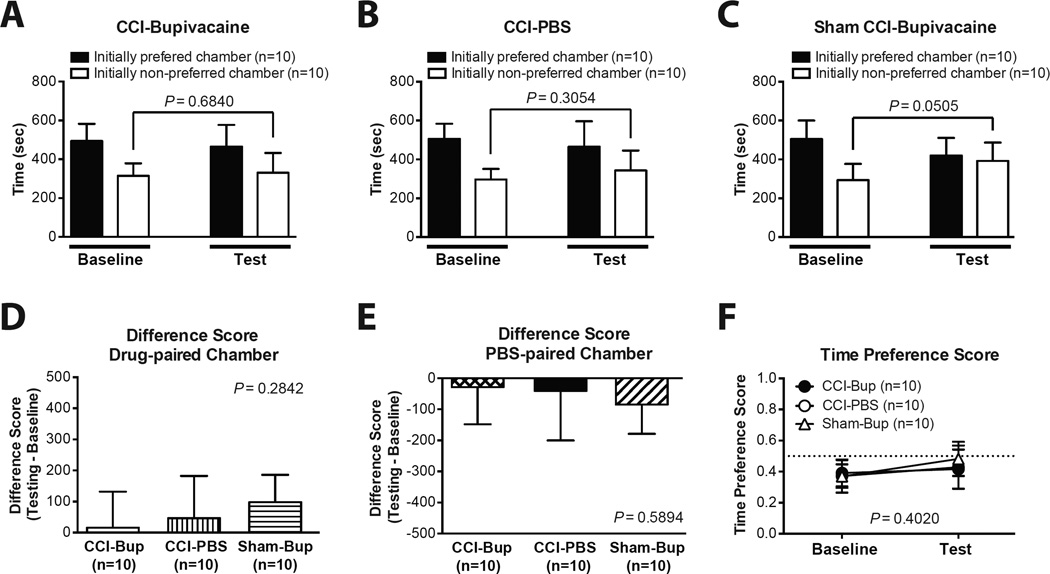
Of 47 rats screened for CPP with CCI, 17 rats were excluded from further study due to their extreme baseline preference/avoidance for a chamber. As shown in Fig. 3, following conditioning, CCI rats did not show a significant preference for the bupivacaine-paired chamber. There was no significant difference in the absolute time spent in the initially non-preferred chamber during baseline vs. test period (Fig. 3A–C). The Difference Scores for the drug-paired (Fig. 3D) and the PBS-paired chambers (Fig. 3E) of the CCI-Bupivacaine group were not significantly different from those of the CCI-PBS and the Sham CCI-Bupivacaine groups. Similarly, the Time Preference Scores for the baseline and the test period of the CCI-Bupivacaine group were not significantly different from those of the CCI-PBS and the Sham CCI-Bupivacaine groups (Fig. 3F).
Fig. 3.
Conditioned place preference in chronic constriction injury (CCI) rats. (A–C) Time spent in each chamber during the baseline and the test period in the CCI-Bupivacaine group (A), CCI-PBS group (B), and Sham CCI-Bupivacaine group (C). (D) The Difference Score for the drug-paired chamber. (E) The Difference Score for the PBS-paired chamber. (F) The Time Preference Score during the baseline and the test period. The results are expressed as mean and standard deviation. CCI-Bup = CCI-bupivacaine group; Sham-Bup = Sham CCI-Bupivacaine group.
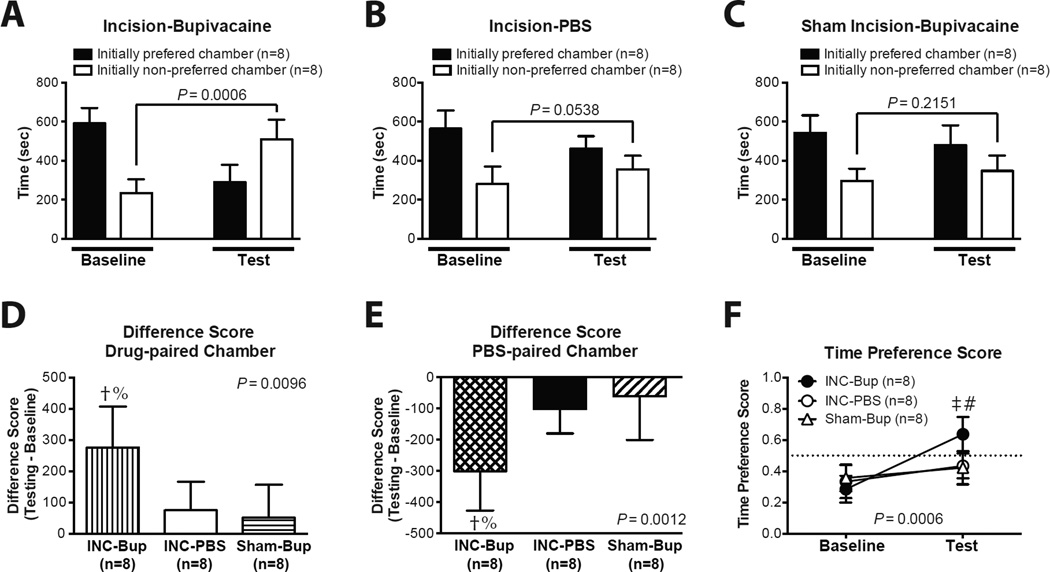
We undertook another CPP experiment using hindpaw incision. Of 32 rats screened for CPP with hindpaw incision, 8 rats were excluded from further study due to their extreme baseline preference/avoidance for a chamber. As shown in Fig. 4, following conditioning, incised rats developed a significant preference for the bupivacaine-paired chamber. In the Incision-Bupivacaine group, following bupivacaine conditioning, animals spent significantly greater time in the initially non-preferred chamber compared to baseline (Fig. 4A). On the other hand, the Incision-PBS group and the Sham-Bupivacaine group did not show any difference in the absolute time spent in the initially non-preferred chamber during baseline vs. test period (Fig. 4B and C). The incised rats spent significantly more time in the bupivacaine-paired chamber (Fig. 4D) and significantly less time in the PBS-paired chamber (Fig. 4E) during the test period. The Difference Scores of the Sham Incision-Bupivacaine groups were not significantly different from those of the Incision-PBS group (Fig. 4D and E). During the baseline period, there was no significant difference in the Time Preference Scores among groups (Fig. 4F). During the test period, the Time Preference Score of the Incision-Bupivacaine group was significantly greater those of the Incision-PBS (P < 0.001) and the Sham Incision-Bupivacaine (P < 0.001) groups (Fig. 4F). Using this biased CPP paradigm, bupivacaine did not show any preference in control rats in either the incision protocol or CCI protocol.
Fig. 4.
Conditioned place preference in incised rats. (A–C) Time spent in each chamber during the baseline and the test period in the Incision-Bupivacaine group (A), Incision-PBS group (B), and Sham Incision-Bupivacaine group (C). (D) The Difference Score for the drug-paired chamber. (E) The Difference Score for the PBS-paired chamber. (F) The Time Preference Score during the baseline and the test period. The results are expressed as mean and standard deviation. †P < 0.01 vs. Incision-PBS group; %P < 0.01 vs. Sham-Bupivacaine group, one-way ANOVA, followed by post-hoc Tukey’s multiple comparison test. ‡P < 0.001 vs. Incision-PBS group; #P < 0.001 vs. Sham-Bupivacaine group, two-way ANOVA with repeated measures on one factor, followed by post-hoc Bonferroni test. INC-Bup = Incision-bupivacaine group; INC-PBS = Incision-PBS group; Sham-Bup = Sham Incision-Bupivacaine group.
Behavioral Observations
Some behavioral features were noted only in rats with established CCI, but not in sham animals. CCI rats generally walked with a limp, and dragging of the affected foot was occasionally noted. In a few CCI rats this was less noticeable. While at rest, the CCI rats often kept the affected leg in a guarded position, with their toes held together in a curled position. They often lied down on their side with the affected leg off the ground or mesh floor. These behavioral features were not quantitatively measured. These findings are in accordance with previously described behavioral patterns following CCI [2; 3].
Electrophysiology Studies
Single unit action potentials were recorded from 113 different neurons. 61 units were from 19 CCI animals, and 52 units were from 19 sham animals. All recorded units had a receptive field located in the left hindlimb corresponding to the side of the CCI surgery. In the sham group, 35 (67%) were classified as WDR, 14 (27%) were classified as HT. In the CCI group 35 (57%) were classified as WDR, 19 (31%) were classified as HT. An additional 3 units (5.7%) in the sham group and 7 (11.5%) in the CCI group had receptive fields in the hindlimb but were not completely classified.
Spontaneous Activity
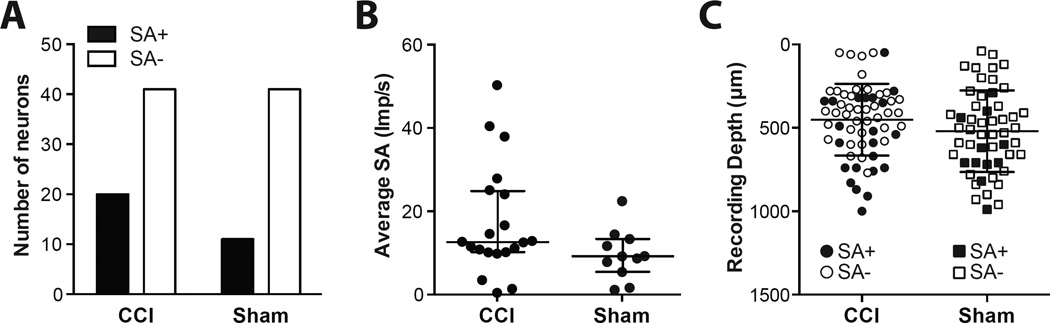
An example of neurons without SA and with SA are shown in in Fig 5A–B. Both neurons’ activities were increased by somatic stimulation of the hindlimb. SA was identified in 20 of 61 neurons (33%) in the CCI group, and in 11of 52 neurons (21%, P = 0.1672) in the sham group (Fig. 6A). In the CCI group, the median rate of SA was 12.6 imp/s compared to 9.2 imp/s in the sham group (Fig. 6B, P = 0.0643).
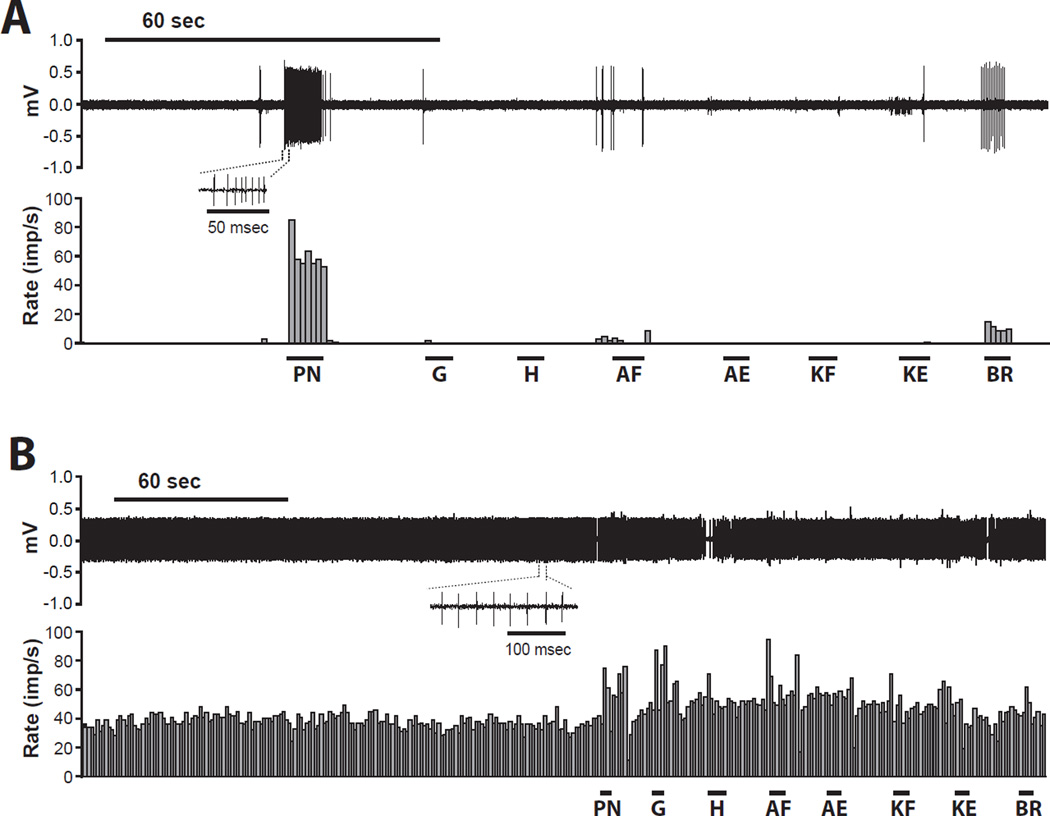
Fig. 5.
Example recordings of dorsal horn neurons without spontaneous activity (A) and with spontaneous activity (B). Both neurons are from the chronic constriction injury (CCI) group. Neuronal responses to various stimuli, including pinch to the hindpaw (PN), gastrocnemius (G), and hamstring muscle (H), followed by ankle flexion (AF), ankle extension (AE), knee flexion (KF), knee extension (KE), and then brushing of the hindlimb (BR) are shown. The upper and lower panels show digitalized oscilloscope trace of the action potentials and the cumulative histogram of the rate of action potentials (bin width = 1 sec), respectively. Imp/s = impulses per second; mV = millivolts.
Fig. 6.
Spontaneous activity of the dorsal horn neurons in the chronic constriction injury (CCI) and the Sham group. (A) Prevalence of dorsal horns neurons with spontaneous activity (SA+) and without spontaneously active (SA−) in the CCI and the sham group. (B) Comparison of rate of spontaneous activity between the CCI and the sham group. Each circle represents average spontaneous activity of a neuron. Bars represent median and whiskers represent interquartile range. (C) Depth for each dorsal horn neuronal recording. Filled and open symbols represent neurons with and without spontaneous activity, respectively. Bars represent mean and whiskers represent standard deviation. SA = spontaneous activity; imp/s = impulses per second; µm= micrometers.
The depths of the neuron recordings from both those with and without SA are shown in Fig. 6C. There was no difference in the depth of all neurons recorded between groups. The average depth of all neurons was 520 ± 34 µm in the sham group, and 451 ± 27 µm in the CCI group (P = 0.1124). Also, there was no difference in the depth of spontaneously active neurons recorded between groups. For the spontaneously active neurons, the average depth was 637 ± 60 µm in the sham group, and 560 ± 53 µm in the CCI group (P = 0.3745).
Effect of Bupivacaine on Spontaneous Activity
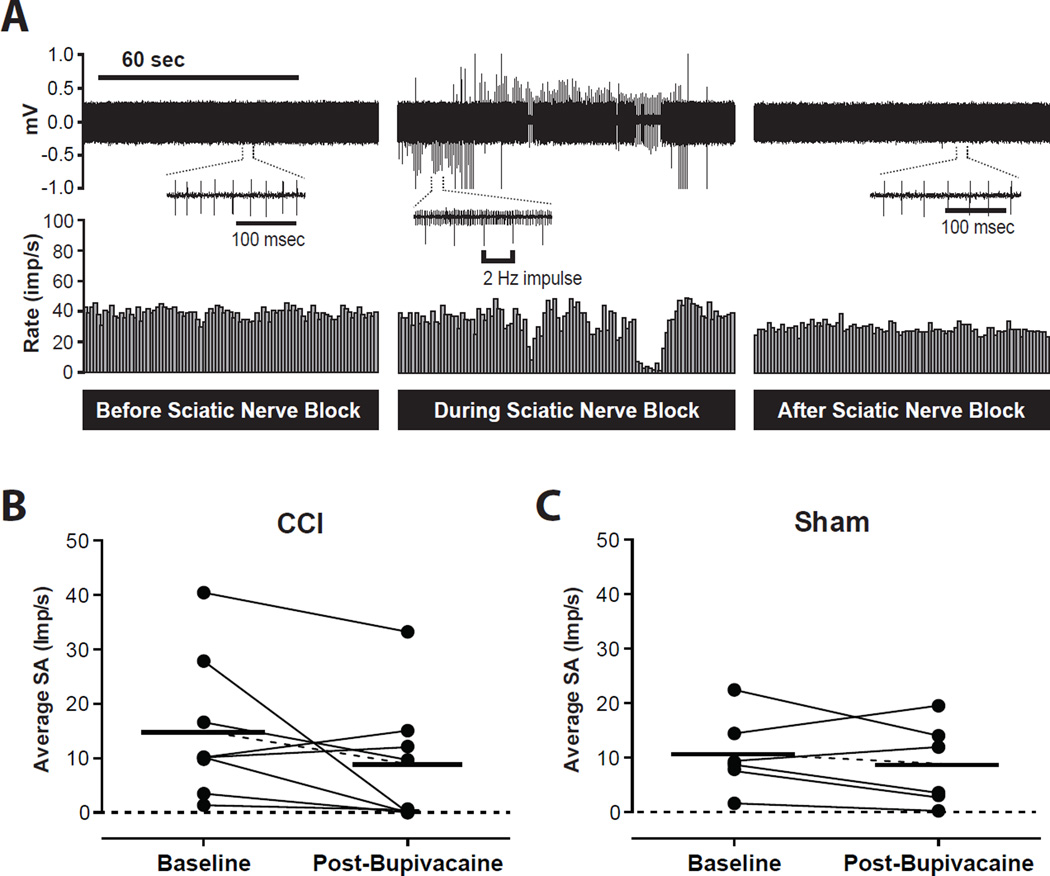
In total, 8 spontaneously active neurons in the CCI group and 6 spontaneously active neurons in the sham group underwent high sciatic nerve block with bupivacaine. An example of action potentials from a neuron with SA before, during and after sciatic nerve block is shown in Figs. 7A. In these selected neurons, prior to bupivacaine injection, the mean rate of SA in the CCI group was 14.9 ± 4.6 imp/s compared to 8.8 ± 4.1 imp/s after injection (P = 0.1316, Fig. 7B). In the sham group, the mean rate of SA was 10.7 ± 2.8 imp/s before bupivacaine injection and 8.7 ± 3.1 imp/s after bupivacaine injection (P = 0.3867, Fig. 7C). There was no significant effect of bupivacaine on spontaneous activity in either group.
Fig. 7.
Effect of high sciatic nerve block with bupivacaine on spontaneous activity (SA) of dorsal horn neurons. (A) Example recording of a dorsal horn neuron with spontaneous activity from the chronic constriction injury (CCI) group. The upper and lower panels show digitalized oscilloscope trace of the action potentials and the cumulative histogram of the rate of action potentials (bin width = 1 sec), respectively. (B and C) Summary of average spontaneous activity before (baseline) and after sciatic nerve block with bupivacaine (Post-Bupivacaine) in the CCI group (B) and in the sham group (C). Each circle represents average spontaneous activity of a neuron. Bars represent mean. Imp/s = impulses per second; mV = millivolts.
After Discharge
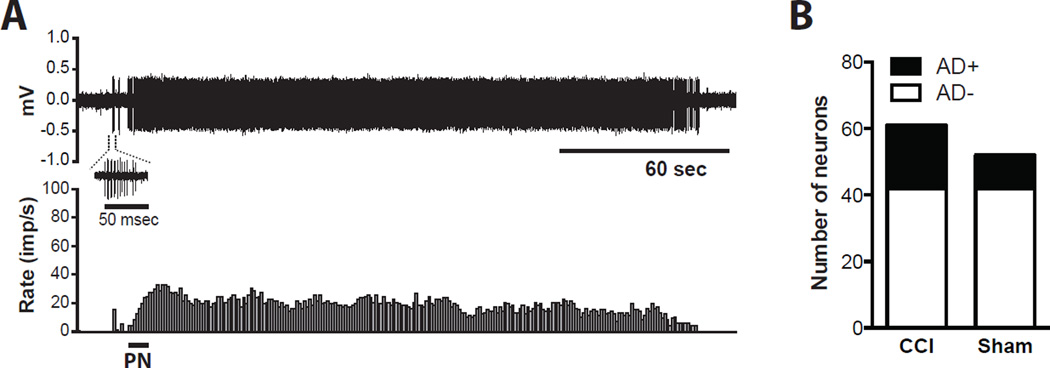
An example of a neuron with after discharge is shown in Fig. 8A. In this neuron, the response to pinch was sustained for approximately 3 min after the stimulus. In the sham-operated rats, 10 of 52 neurons (19%) had an episode of after discharge after somatic testing; 19 of 61 neurons (31%) had an episode of after discharge in the CCI group (Fig. 8B, P = 0.1483). The characteristics of the after discharge were similar between groups.
Fig. 8.
Dorsal horn neurons with after discharge in the chronic constriction injury (CCI) and the Sham group. (A)Example recording of a dorsal horn neuron with after discharge. The neuron in this example is from the CCI group. The upper and lower panels show digitalized oscilloscope trace of the action potentials and the cumulative histogram of the rate of action potentials (bin width = 1 sec), respectively. (B) Prevalence of dorsal horns neurons with after discharge (AD+) and without after discharge (AD−) in the CCI and the sham group. PN = pinch to the hindpaw; imp/s = impulses per second; mV = millivolts; AD = after discharge.
Discussion
In this study, our focus was CPP and spontaneous activity in dorsal horn neurons in CCI rats. While CCI produced nocifensive responses to mechanical stimuli, CPP could not be elicited in the CCI rats using nerve block. We also could not demonstrate increased magnitude or prevalence of dorsal horn neuron spontaneous activity in CCI rats. On the other hand, CPP could be elicited after hindpaw incision. After hindpaw incision, we have previously shown that both the proportion of dorsal horn neurons with spontaneous activity and the rate of this spontaneous activity were increased [34; 36; 37]. Our data does not support CCI as an animal model that can be used to study ongoing spontaneous nociception driven by the peripheral injury.
Behavioral studies
CCI failed to induce a CPP following high sciatic nerve block with bupivacaine 14 days following CCI (Fig. 3). On the other hand, sciatic nerve block after hindpaw incision produced CPP on postoperative day 1 (Fig. 4). This same incision model has been shown to associate primary afferent and dorsal horn neuron spontaneous activity and guarding behavior on postoperative day 1 [34; 35]. In addition, Navratilova et al. have shown that CPP can be elicited in rats after hindpaw incision on postoperative day 1 [22].
We used a sciatic nerve block that was injected proximal to the CCI injury to block any injury signal from the CCI site. We utilized a combination of anatomical landmarks [29] and motor response to electrical stimulation to localize the proximal sciatic nerve. This technique allowed us to produce reliable nerve blocks because we observed new motor deficits after emergence from anesthesia following nerve block. We also noted that motor responses were more difficult to elicit during nerve block in CCI rats compared to the sham rats, likely due to partial denervation by CCI [12; 21].
We used nocifensive withdrawal response to mechanical stimuli to demonstrate that CCI produced nociceptive behaviors in our laboratory. It is controversial whether to use withdrawal threshold or nocifensive responses to mechanical stimuli to study mechanical hypersensitivity after nerve injury [10; 19; 20]. We preferred nocifensive responses rather than simple withdrawal because the nocifensive behaviors are associated with aversion in behavioral studies [10].
Several studies have suggested unprovoked pain-related behaviors in CCI rats. Modifications in the spontaneous postures of the affected hind paw at rest have been described for instance [2]. Others have suggested spontaneous pain is possibly present in this model, based on modestly impaired weight gain and their interpretation of other unquantified findings [3]. However, these behavioral changes were not tested against analgesic agents. Also, the altered posture may reflect changes that are unrelated to pain, such as altered motor control and learned responses to avoid weight bearing on injured hindlimb.
Dorsal horn neuron recording
Our electrophysiology data are associated with the negative CPP data. This is in accordance with previous data suggesting that CCI does not significantly change the overall number of spontaneously active neurons recorded from the lumbar enlargement [18]. In this previous report [18], when spontaneous activities from all frequencies were grouped together, the total number of spontaneously active neurons was 23% in both groups. While the proportion of neurons with spontaneous activity with higher frequencies (greater than 7 imp/sec) was increased in the nerve-injured animals, the overall proportion of spontaneously active neurons was similar. In this study, the investigators did not utilize somatic fields as criteria for recording dorsal horn neuron activities, and any dorsal horn neuron was studied regardless of whether there was any receptive field. Our study was different in that we only studied neurons with at least some receptive field in the hindleg. Our data showed that blocking the peripheral input proximal to the site of injury, using anatomic and nerve stimulation endpoints, did not significantly change the rate of the spontaneous activity in the CCI rats vs. the sham group. We also note that our search criteria included a hindlimb somatic receptive field in what may be a partial denervation model [12]. The cause of the small reduction in spontaneous activity after nerve block in both groups is not known. It may be related to leg position producing activity during recording, for example.
It is possible that spontaneous pain-related behaviors are present but not evident using CPP because the ectopic discharges are generated in pain transmitting neurons proximal to the injection site. This is less likely though, considering that neither the magnitude nor the prevalence of dorsal horn neuron spontaneous activity was significantly increased following CCI. These electrophysiological characteristics of CCI rats are different from previously reports using the spinal ligation model; both the magnitude and the prevalence of dorsal horn neuron spontaneous activity was significantly increased 7–17 days following spinal nerve ligation [8; 28; 29]. Perhaps such dissociations suggest that different underlying mechanisms may be involved in different neuropathic pain models, depending on the type and severity of nerve damage. Overall, our findings suggest that CCI as a neuropathic pain model does not produce spontaneous pain driven from the injury.
Clinical perspective
From a clinical perspective, spontaneous pain is a major problem in patients with nerve injury. Patients commonly present with symptoms of ongoing and/or paroxysmal pain [5; 13; 27], in addition to allodynia and hyperalgesia. Spontaneous pain may be an important clinical distinction to note when evaluating patients with neuropathic pain, as learning how to best treat spontaneous pain may lead to improved pain outcomes. While there are many therapeutic interventions to consider, including physical therapy, medications, surgical interventions, and neuromodulation, one important approach to improve therapeutic interventions for neuropathic pain is to recognize that pain relief may be from reduction in spontaneous pain, hypersensitivity or both. This phenotyping has been emphasized by the European consortium on neuropathic pain [1; 9].
Pain has a sensory and an affective component. This affective component of pain contributes to patients’ response to certain environmental or emotional cues [11; 32]. One of the challenges in the current method of studying neuropathic pain in animal models is evaluation of spontaneous pain. While evoked responses in the preclinical models of neuropathic pain are well characterized [7; 16; 26; 33], determining whether spontaneous pain is present has been more challenging. CPP is one such method of testing that has been shown to indicate the presence of spontaneous pain [16]. CPP may also assess the affective component of pain, which often develops in concordance with spontaneous pain.
Conclusion
In conclusion, this study demonstrates that approximately 14 days following CCI, dorsal horn neuron activity is not significantly increased compared to the sham-operated group. Also there was no change in dorsal horn neuron spontaneous activity following high sciatic nerve block with bupivacaine. CPP to a proximal analgesic nerve block was not evident in CCI animals 14 days following injury. This suggests that CCI as a neuropathic pain model does not produce spontaneous nociception from the injury, and should not be used as a model to measure effects of treatment of spontaneous pain driven by the peripheral input.
Acknowledgments
The authors acknowledge Alberto Subieta B.S. for performing CPP in rats with hindpaw incision.
This work was supported by the Samir D. Gergis Endowment.
Footnotes
The authors do not have any conflict of interest.
References
- 1.Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2010;17(9):e1113–e1188. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 2.Attal N, Jazat F, Kayser V, Guilbaud G. Further evidence for 'pain-related' behaviours in a model of unilateral peripheral mononeuropathy. Pain. 1990;41(2):235–251. doi: 10.1016/0304-3959(90)90022-6. [DOI] [PubMed] [Google Scholar]
- 3.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJM. Management of Diabetic Peripheral Neuropathy. Clinical Diabetes. 2005;23(1):9–15. [Google Scholar]
- 5.Boulton AJM. Management of Diabetic Peripheral Neuropathy. Clincial Diabetes. 2005;52(1):77–92. [Google Scholar]
- 6.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64(3):493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman V, Suzuki R, Dickenson AH. Electrophysiological characterization of spinal neuronal response properties in anaesthetized rats after ligation of spinal nerves L5–L6. The Journal of physiology. 1998;507(Pt 3):881–894. doi: 10.1111/j.1469-7793.1998.881bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, Haanpaa M, Jensen TS, Serra J, Treede RD. EFNS guidelines on neuropathic pain assessment: revised 2009. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2010;17(8):1010–1018. doi: 10.1111/j.1468-1331.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 10.Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101(2):476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98(14):8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol. 1992;68(3):734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- 13.Kelly KG, Cook T, Backonja MM. Pain ratings at the thresholds are necessary for interpretation of quantitative sensory testing. Muscle Nerve. 2005;32(2):179–184. doi: 10.1002/mus.20355. [DOI] [PubMed] [Google Scholar]
- 14.Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113(2):200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- 15.King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152(9):1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12(11):1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohane DS, Yieh J, Lu NT, Langer R, Strichartz GR, Berde CB. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology. 1998;89(1):119–131. doi: 10.1097/00000542-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Laird JM, Bennett GJ. An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental peripheral neuropathy. J Neurophysiol. 1993;69(6):2072–2085. doi: 10.1152/jn.1993.69.6.2072. [DOI] [PubMed] [Google Scholar]
- 19.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacological reviews. 2001;53(4):597–652. [PubMed] [Google Scholar]
- 20.Mogil JS. Animal models of pain: progress and challenges. Nature reviews Neuroscience. 2009;10(4):283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 21.Munger BL, Bennett GJ, Kajander KC. An experimental painful peripheral neuropathy due to nerve constriction. I. Axonal pathology in the sciatic nerve. Exp Neurol. 1992;118(2):204–214. doi: 10.1016/0014-4886(92)90037-q. [DOI] [PubMed] [Google Scholar]
- 22.Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012;109(50):20709–20713. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Molecular pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pertovaara A, Kontinen VK, Kalso EA. Chronic spinal nerve ligation induces changes in response characteristics of nociceptive spinal dorsal horn neurons and in their descending regulation originating in the periaqueductal gray in the rat. Exp Neurol. 1997;147(2):428–436. doi: 10.1006/exnr.1997.6555. [DOI] [PubMed] [Google Scholar]
- 25.Pogatzki EM, Vandermeulen EP, Brennan TJ. Effect of plantar local anesthetic injection on dorsal horn neuron activity and pain behaviors caused by incision. Pain. 2002;97(1–2):151–161. doi: 10.1016/s0304-3959(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 26.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139(2):243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Rowbotham MC. Mechanisms of neuropathic pain and their implications for the design of clinical trials. Neurology. 2005;65(12 Suppl 4):S66–S73. doi: 10.1212/wnl.65.12_suppl_4.s66. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki R, Chapman V, Dickenson AH. The effectiveness of spinal and systemic morphine on rat dorsal horn neuronal responses in the spinal nerve ligation model of neuropathic pain. Pain. 1999;80(1–2):215–228. doi: 10.1016/s0304-3959(98)00208-5. [DOI] [PubMed] [Google Scholar]
- 29.Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995;82(4):1013–1025. doi: 10.1097/00000542-199504000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Tzschentke TM, Schmidt WJ. N-methyl-D-aspartic acid-receptor antagonists block morphine-induced conditioned place preference in rats. Neuroscience letters. 1995;193(1):37–40. doi: 10.1016/0304-3940(95)11662-g. [DOI] [PubMed] [Google Scholar]
- 31.Vandermeulen EP, Brennan TJ. Alterations in ascending dorsal horn neurons by a surgical incision in the rat foot. Anesthesiology. 2000;93(5):1294–1302. doi: 10.1097/00000542-200011000-00024. discussion 1296A. [DOI] [PubMed] [Google Scholar]
- 32.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135(1–2):7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979;7(2):103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Brennan TJ. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144(3):329–339. doi: 10.1016/j.pain.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology. 2010;112(1):153–164. doi: 10.1097/ALN.0b013e3181c2952e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Richebe P, Brennan TJ. Separate groups of dorsal horn neurons transmit spontaneous activity and mechanosensitivity one day after plantar incision. Eur J Pain. 2009;13(8):820–828. doi: 10.1016/j.ejpain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Zahn PK, Brennan TJ. Incision-induced changes in receptive field properties of rat dorsal horn neurons. Anesthesiology. 1999;91(3):772–785. doi: 10.1097/00000542-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 38.Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain. 2005;114(3):499–510. doi: 10.1016/j.pain.2005.01.018. [DOI] [PubMed] [Google Scholar]