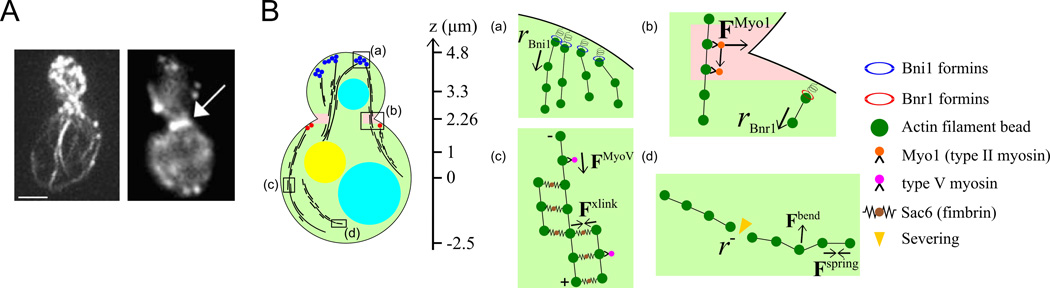
Figure 1. Model description.
(A) Experimental images of phalloidin-stained actin cables (left, reprinted from [Liu et al. 2012]) and cytokinetic ring (right, arrow, reprinted from [Tolliday et al. 2002] with permission from Elsevier) in budding yeast. Scale bar: 2 µm (B) A schematic diagram shows the geometric shape we use for budding yeast, with one sphere centered at (0,0,3.3) with radius 1.5 µm (bud) and another centered at (0,0,0) with radius 2.5 µm (mother). The two spheres intersect at z = 2.26 µm to form the neck. The two cyan and one yellow spheres represent two vacuoles and the nucleus, respectively. (Right) Schematic of implemented simulation mechanisms. (a) Beads representing Bni1 formins placed at the bud tip (blue) polymerize actin filaments (dark green beads). (b) Within the ring-shaped area around the neck (pink), the filament beads experience forces in both tangential and outward directions, representing binding to Myo1 (orange). At the neck, beads representing Bnr1 formins (red) are placed on the mother cell boundary, right below the neck where they polymerize actin filaments at a faster speed compared to Bni1. (c) Myosin V motors (violet) bind to the actin filaments at a certain rate and exert forces toward the barbed ends. An attractive interaction (brown), representing cross-linking is established when two actin filament beads come close to one another. This interaction depends on the relative orientation of the filaments. (d) Connections between filament beads are severed (yellow) with a rate that depends on the age of the connecting spring, representing age-dependent filament severing by cofilin and cofactors.