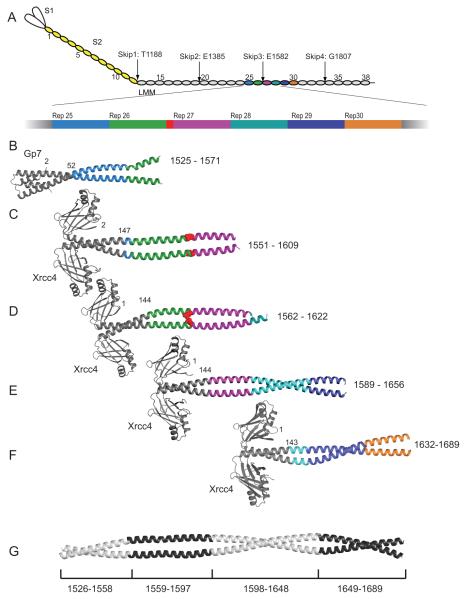
Figure 1.
Structures of myosin fusion proteins and preliminary composite model. (A) A representation of myosin in which each 28 amino acid repeat of the C-terminal coiled-coil is an oval. S1, S2, and LMM are colored white, yellow and grey respectively. The numbering is shown for every fifth repeat and the positions of the skip residues are indicated. Repeats 25 through 30 are colored differently and the third skip residue, E1582, is shown in red. The fusion proteins are colored in grey while the myosin repeats are colored as in panel A for (B) Gp7-L1526-E1571, (C) Xrcc4-L1551-N1609, (D) Xrcc4-Q1562-L1622. (E) Xrcc4-H1590-L1657, (F) Xrcc4-A1632-R1689 and (G) A simple composite model for L1526-R1689 of human cardiac β-myosin. This was assembled from four of the five overlapping structures taken from Gp7-L1526-E1571, Xrcc4-L1551-N1609, Xrcc4-H1590-L1657, and Xrcc4-A1632-R1689. The residues incorporated from each structure are listed below. The coordinates for Xrcc4-L1551-N1609 were taken from the RCSB with accession 4XA4. Figures 1, 2, 3, 4, 5, 8, 9, and 10 were prepared in part with Pymol (http://www.pymol.org/).