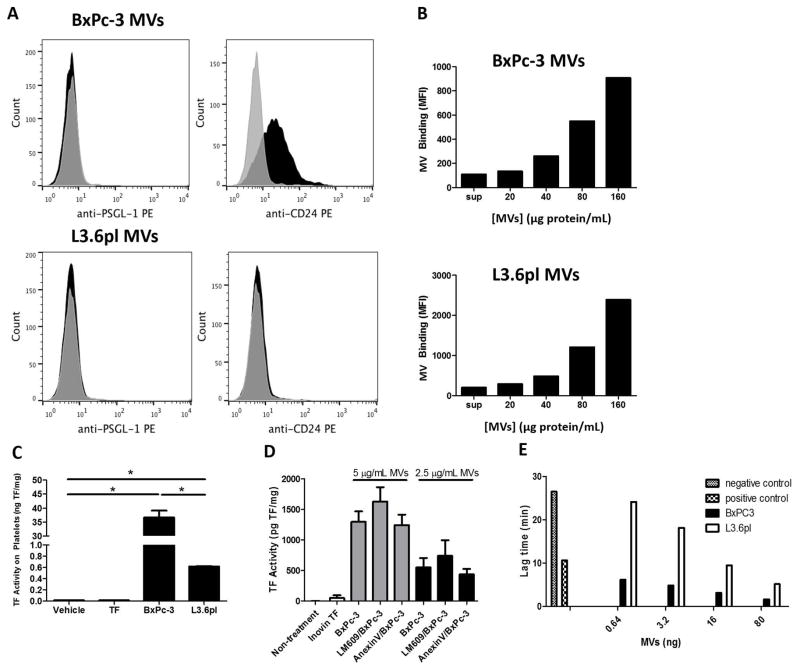
Figure 2. Binding of TF positive tumor microvesicles to platelets.
(A) Expression of the P-selectin ligands PSGL-1 and CD24 was evaluated on MVs isolated from BxPc-3 and L3.6pl cells by flow cytometery. Representative histograms are shown. (B) BxPc-3 and L3.6pl TMVs were stained with 4 μM CFSE fluorescent dye followed by 2 wash steps at 20,000 x g for 20 minutes. The indicated concentrations of stained TMVs or washed supernatant were incubated with human platelets in the presence of 1:100 diluted human plasma for 15 minutes at 37°C in the presence of anti-human CD41a-PE. TMV-platelet binding was quantified by measuring the percentage of CFSE-positive platelet events by flow cytometry. A representative experiment of 3 independent experiments is shown. (C) Washed human platelets were incubated with 10 μg of BxPc-3 MVs (20 pg TF), 10 μg of L3.6pl MVs (0.4 pg TF), 0.5 pg of Innovin TF, or vehicle control for 15 minutes at 37°C. Platelets were then washed and cellular TF activity was determined to quantify the delivery of TMV TF to platelets. *P<0.0001, n=3. TF activity was normalized to total protein. (D) Incubation of platelets with either annexin V (2.5 μg/mL final concentration) or the anti-αvβ3 antibody LM609 (1:100 dilution) at room temperature for 15 minutes did not reduce the amount of TF delivered from either 5 or 2.5 μg/mL of BxPc-3 MVs. Data were analyzed by one-way ANOVA with Bonferroni posttests comparing indicated pairs of data. (E) BxPc-3 and L3.6pl MVs led to a dose-dependent shortening of lag time in a calibrated automated thrombogram with platelet-rich plasma. The PRP trigger reagent (1 pM TF) was added to the positive control and buffer was added to the negative control. Data are shown from one donor representative of 3 different donors.