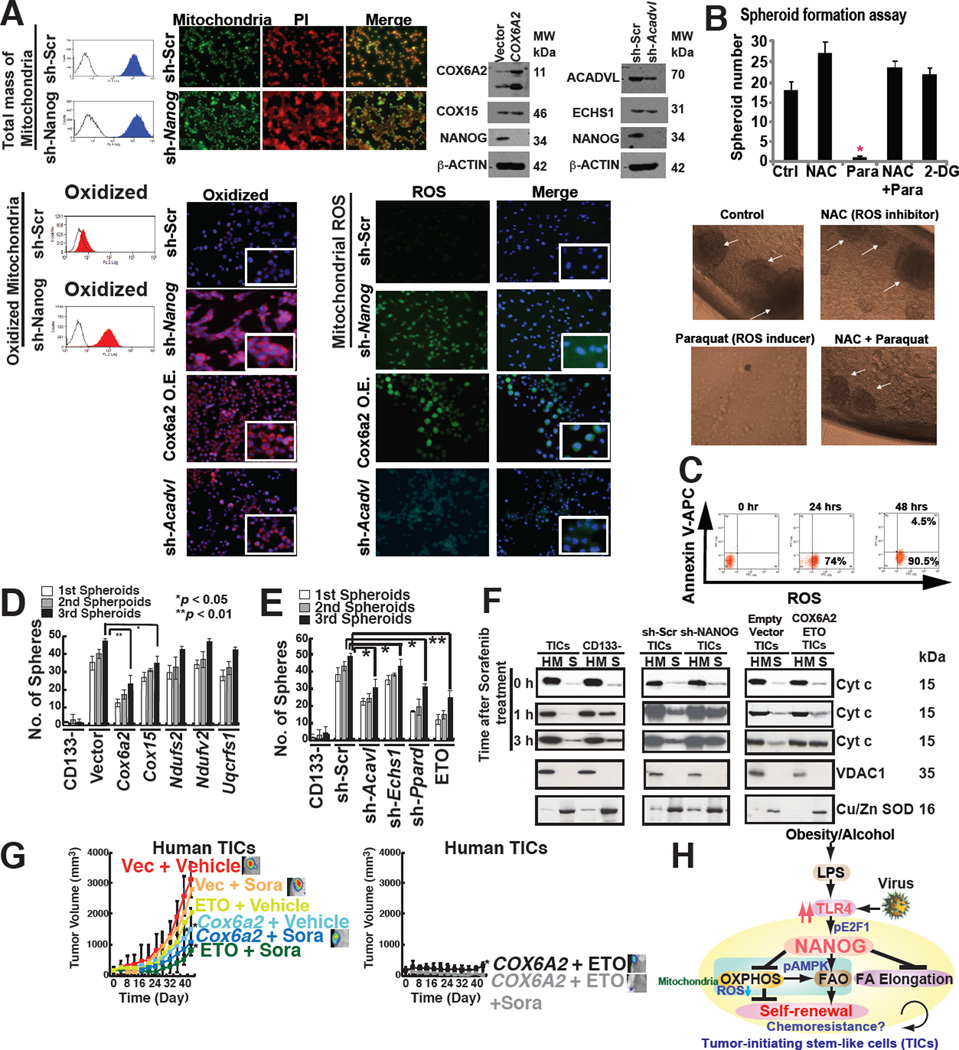
Fig. 7. NANOG orchestrated TIC oncogenic and therapeutic resistance mechanisms via mitochondrial metabolic reprogramming.
(A) Mitochondrial ROS production increased in sh-Nanog TICs, but total mitochondrial levels were unchanged in TICs compared to sh-Nanog TICs.
(B) ROS inducer Paraquat (Para), but not ROS scavenger (NAC), inhibited spheroid formation, but minimal cell death induction was observed (C).
(D) Restoration of OXPHOS genes in TICs promoted self-renewal ability.
(E) Silencing OXPHOS genes and FAO genes inhibited spheroid formation.
(F) Mitochondrial cytochrome c release was increased by the combination of sorafenib and ETO treatment or overexpression of Cox6a2 in TICs. Cytochrome c release from mitochondria was analyzed by immunoblotting of the cytosol (soluble fraction) and mitochondria-rich (heavy membrane: HM) fractions of the cell lysates. TICs and CD133(−) cells transduced with sh-Nanog were lysed and fractionated into purified heavy membrane (HM) and cytosolic (S) fractions. The fractions were then probed for cytochrome c (Cyt c), VDAC1 and Cu/Zn SOD.
(G) Overexpression of Cox6a2 and ETO treatment abrogated drug-resistance and reduced tumor growth.
(H) A summary diagram depicting the proposed roles of TLR4/NANOG for metabolic reprogramming and genesis of TICs in liver oncogenesis due to alcohol and HCV. NANOG-induced chemotherapy-resistance occurred via mitochondrial metabolic reprogramming (suppression of mitochondrial OXPHOS and promotion of FAO).