Abstract
Scope
Nobiletin (NBT) is a major citrus flavonoid with various health benefits. Herein, we investigated the colon cancer chemopreventive effects of NBT and its colonic metabolites in a colitis-associated colon carcinogenesis mouse model as well as in human colon cancer cell models.
Methods and results
In azoxymethane (AOM)/dextran sulfate sodium (DSS)-treated mice, oral administration of NBT effectively decreased both incidence and multiplicity of colonic tumors. NBT showed significant anti-proliferative, pro-apoptotic and anti-inflammatory effects in the mouse colon. HPLC analysis revealed that oral administration of NBT resulted in high levels of metabolites, i.e. 3′-demethylnobiletin (M1), 4′-demethylnobiletin (M2), and 3′, 4′-didemethylnobiletin (M3) in the colonic mucosa. In contrast, the colonic level of NBT was about 20-fold lower than the total colonic level of three metabolites. Cell culture studies demonstrated that the colonic metabolites of NBT significantly inhibited the growth of human colon cancer cells, caused cell cycle arrest, induced apoptosis, and profoundly modulated signaling proteins related with cell proliferation and cell death. All of these effects were much stronger than those produced by NBT alone.
Conclusions
Our results demonstrated that oral administration of NBT significantly inhibited colitis-associated colon carcinogenesis in mice, and this chemopreventive effect was strongly associated with its colonic metabolites.
Keywords: chemoprevention, colitis, colon carcinogenesis, metabolites, nobiletin
1. Introduction
Colon cancer is one of the major causes of cancer death and poses a serious threat to human health. It is well known that there is a strong association between inflammation and multiple types of cancers including colon cancer [1]. Both referral center studies and population-based studies have demonstrated that patients with inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease are at an significantly increased risk of colon cancer as compared to the general population [2]. Inflammation promotes carcinogenesis by various mechanisms including enhancing proliferation of malignant cells, promoting angiogenesis, boosting tumor metastasis, and altering tumor response to chemotherapeutic drugs [1]. Accumulating evidence showed that certain dietary compounds have anti-inflammatory and anti-carcinogenic effects, and they can be potentially utilized as chemopreventive agents against inflammation-associated colon carcinogenesis [3].
Polymethoxyflavones (PMFs) is a unique class of flavonoids mainly found in citrus fruits [4]. Nobiletin (5,6,7,8,3′,4′-hexamethoxyflavone, NBT) is a major citrus PMF. Previous studies have demonstrated a wide range of beneficial activities of NBT, including anti-inflammation [5–7], anti-cancer [8–12], and anti-atherosclerosis [13]. NBT has shown various protective effects against different cancers in animal models, such as gastric [8], colon [10–12], and prostate [12] cancers. In particular, dietary administration of NBT was shown to suppress colon carcinogenesis in different rodent models [10–12]. Suzuki et al. reported that NBT inhibited AOM-induced colon carcinogenesis in rats [10]. Miyamoto et al. demonstrated that NBT inhibited AOM/DSS-induced colon carcinogenesis in mice, and this protective effect was associated with down-regulation of leptin [11]. Tang et al. showed that NBT suppressed PhIP-induced formation of aberrant crypt foci in the rat colon [12]. Together, these studies suggested that dietary administration of NBT generally inhibited chemically induced colon carcinogenesis in rodents.
A growing body of evidence suggested that biotransformation played critical roles in the biological activities of orally administered compounds [14, 15]. The metabolites generated in the body by the biotransformation may have more potent bioactivities in comparison with the parent compound [16, 17]. Moreover, oral administration of a parent compound may result in higher levels of metabolites than the parent compound in certain tissues. Therefore, under the aforementioned circumstance, the metabolites, rather than the ingested parent compound, may exhibit dominant biological effects in those tissues [17]. Previously, the colon cancer chemopreventive effects of NBT observed in rodent models were ascribed to NBT itself [10–12]. There is no detailed information available about the role of biotransformation in the chemopreventive effects of NBT in the colon. To better understand the biological activities of NBT, we systematically investigated the inhibitory effects of dietary NBT on colitis-associated colon carcinogenesis in AOM/DSS-treated CD-1 mice; identified and quantified major colonic metabolites of NBT in the mice, and elucidated the inhibitory effects of these metabolites on human colon cancer cells.
2. Materials and methods
2.1 Animals, diets and experimental procedure
The protocol for the animal experiment was approved by Institutional Animal Care and Use Committee of the University of Massachusetts Amherst (# 2014-0079). The male CD-1 mice (5 week old) were obtained from Charles River Laboratories (Wilmington, MA, USA). Upon arrival, the mice were kept in a temperature-controlled animal room (23°C) with humidity of 65–70%, fixed day/night cycle (12 h light), and free access to water and AIN93G diet for 2 week for acclimation [18]. Mice were then randomly divided into three groups (negative control group, positive control group and NBT group, 20 mice each) and were given one intraperitoneal injection of AOM (12 mg·kg−1 body weight) in saline (negative control group received same volume of saline. After 1 week, 1.5% DSS (molecular weight: 36,000–50,000, International Lab, Chicago, IL, USA) was administered in the drinking water for 4 days followed by 1 week of pure water for recovery, and this cycle was repeated four times (negative control group receive regular drinking water without DSS). Negative and positive control groups were fed with AIN93G diet, while NBT group was fed with AIN93G diet containing NBT (0.05 wt% in diet) starting at one week after the AOM injection until the end of study. All mice were sacrificed by CO2 asphyxiation at 20 week after AOM injection. The liver and spleen were removed and weighed. After measurement of the length, the entire colons were opened longitudinally, rinsed with phosphate-buffered saline (pH 7.4). The whole colon weight was measured and the colons were inspected under a dissection microscope. Number and size of tumors were measured using an ocular micrometer. The sizes of tumors were determined by the following formula: tumor volume (mm3) = L × W2/2, where L is the length and W is the width of the tumor [19]. Then the colons were cut into two pieces longitudinally. Half of the colon was fixed in 10% buffered formalin (pH 7.4) for 24 h for further histopathological and immunohistochemical analysis. The other half of the colon was stored at −80°C for ELISA, qRT-PCR, and HPLC analysis.
2.2 Histopathological and immunohistochemical analysis
Formalin fixed colon tissues were processed for paraffin-embedding, sectioning, and haematoxylin and eosin (H&E) staining as we previously described [20]. Based on H&E staining, histological alterations such as mucosal ulceration, dysplasia and carcinoma were evaluated under a microscope (100×) according to the criteria previously reported [21]. Carcinoma was defined as a high-grade dysplasia of colonic mucosa that had invaded beyond the muscularis mucosa and into the submucosa. Immunohistochemical analysis was conducted on the colon tissue sections as we previously described [20, 22]. Cell proliferation in the colonic tissue was measured by staining with the antibodies against proliferating cell nuclear antigen (PCNA). Apoptotic cells were determined by staining with antibodies against cleaved caspase-3. Antibodies were obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA).
2.3 Enzyme-linked immunosorbent assay (ELISA) and real-time qRT-PCR Analysis
Colonic mucosa were scraped and homogenized in a phosphate buffer solution containing 0.4 M NaCl, 0.05% Tween-20, 0.5% BSA, 0.1 mM benzethonium, and 1% protease inhibitor cocktail (Boston Bioproducts, Ashland, MA, USA). The homogenates were centrifuged at 10,000g for 30 min at 0°C. The supernatant was used for quantification of cytokines, i.e. interleukin 1β (IL-1β), interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) by ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Real-Time qRT-PCR analysis was conducted as previously described [23]. The primer pairs were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA) with the following primers: IL-1β F: 5′-ACCTGCTGGTGTGTGACGTT-3′, R: 5′-TCGTTGCTTGGTTCTCCTTG-3′; IL-6 F: 5′-GAGGATACCACTCCCAACAGACC-3′, R: 5′-AAGTGCATCATCGTTGTTCATACA-3′A; TNF-α F: 5′-AGCACAGAAAGCATGATCCG-3′, R: 5′-CTGATGAGAGGGAGGCCATT-3′; β-actin F: 5′-AAGAGAGGCATCCTCACCCT-3′, R: 5′-TACATGGCTGGGGTGTTGAA-3′ [24]. The copy number of each transcript was calculated with respect to the β-actin copy number, using the 2−ΔΔCt method [25].
2.4 Determination of colonic levels of NBT and its metabolites by HPLC
Colonic mucosa samples were homogenized in PBS and extracted with equal volume of ethyl acetate for three times. Pooled ethyl acetate fractions were dried under vacuum and dissolved in 50% methanol. Identification and quantification of NBT and its metabolites were carried out by an HPLC with an electrochemical detector using our previously published method [26, 27]. NBT, M1, M2, and M3, with purity greater than 98%, were used as external standards to identify and quantify NBT, M1, M2, and M3. Tangeretin was used as an internal standard. NBT and tangeretin were from Sigma-Aldrich (St. Louis, MO, USA). M1, M2, and M3 were synthesized as described previously [26–29].
2.5 Analysis of cell viability, cell cycle and apoptosis
Assays for cell viability, cell cycle and apoptosis were conducted as we previously described [30–32]. In brief, human colorectal cancer cells, HCT116 and HT29 (ATCC, Manassas, VA, USA) were seeded in 96-well plates. After 24 h, cells were treated with serial concentrations of NBT and its metabolites, and the cell viability was quantified by MTT method [30–32]. HCT116 and HT29 cells were seeded in 6-well plates for cell cycle and apoptosis analysis. After 24 h of incubation for cell attachment, cells were treated with serial concentrations of NBT and its metabolites in serum complete media. After the treatment, media containing any floating cells were harvested and combined with adherent cells that were detached by brief trypsinization. Cell pellets were washed with 1 mL of ice-cold PBS then subject to cell cycle and apoptosis analysis by flow cytometry as we described previously [30–32]. DMSO was used as vehicle to deliver NBT and its metabolites to the cells. The final concentration of DMSO in all experiments was 0.1% v/v in cell culture media.
2.6 Immunoblotting
Cells were seeded in 150 mm culture dishes. After 24 h of incubation for cell attachment, cells were treated with NBT (40 μM), M1 (40 μM), M2 (40 μM) or M3 (20 μM). After another 24 or 48 h of incubation, cells were washed with ice-cold PBS, and collected with cell scrapers. Whole cell lysates were prepared and then subjected to Western blotting analysis as we previously reported [23, 30–32]. Antibodies for p21Cip1/Waf1, cyclin D1, cleaved caspase-3, cleaved caspase-9, and cleaved poly ADP ribose polymerase (PARP) were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-β-actin antibody was from Sigma-Aldrich (St. Louis, MO, USA).
2.7 Statistical analysis
All data were presented as mean ± SD. Student’s t-test was used to test the mean difference between two groups. Analysis of variance (ANOVA) model was used for the comparison of differences among three or more groups. A p value <0.05 was considered to be statistically significant.
3. Results and discussion
3.1 Oral administration of NBT decreased the incidence and multiplicity of colonic tumors in AOM/DSS-treated mice
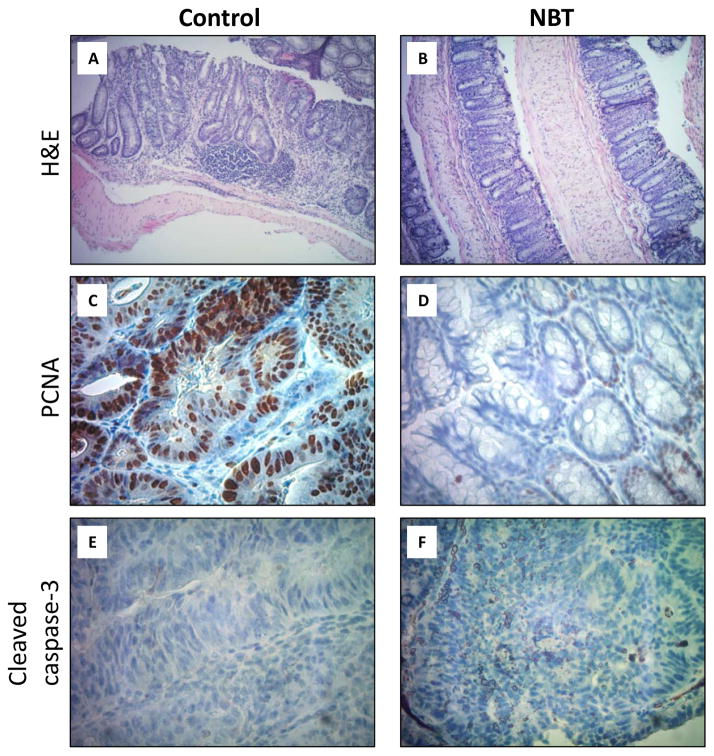
AOM/DSS-treated mice were used to determine chemopreventive effects of dietary NBT (0.05 wt% in AIN93G diet) on colitis-associated colon carcinogenesis. Injection of a colon carcinogen AOM in combination with cyclic administration of DSS in drinking water resulted in the development of colitis, colorectal dysplasia, and cancer [33]. Body weight was monitored twice a week as an indicator of potential toxicity of NBT (significant decrease in body weight may be caused by general toxicity from dietary treatment with NBT). The results showed that no difference was found between NBT-treated group and two control groups during the whole experimental period (final body weights were shown in the Table 1). There was no difference in the weight of liver and spleen between the NBT-treated group and two control groups (Table 1), and no apparent behavioral or appearance difference was observed either, suggesting no noticeable toxic effects caused by dietary feeding of NBT to the mice. Colon weight/length ratio is correlated with the severity of colitis and therefore is an indicator of levels of inflammation in the colon [34]. As shown in Table 1, compared to the positive control group, dietary treatment with NBT significant prevented the shortening of colon length and reduced the elevated colon weight/length ratio caused by AOM/DSS. The colon length and colon weight/length ratio of NBT-treated group were similar to the negative control group. The AOM/DSS treatment in the positive control group resulted in 100% incidence of colon tumors and 4.97 ± 1.01 colonic tumors per mouse (negative control group showed no tumor). Dietary administration of NBT significantly decreased the tumor incidence by 40% and tumor multiplicity by 71% (4.97 ± 1.01 vs. 1.45 ± 0.60). H&E staining showed that the colonic mucosa of the control group presented typical inflammatory and cancerous characteristics such as formation of dysplasia, distortion of crypts structure and infiltration of inflammatory cells (Figure 1A). In contrast, the colonic mucosa of NBT-treated group appeared to largely maintain the histological characteristics of normal mucosa (Figure 1B). These results demonstrated that NBT effectively inhibited AOM/DSS-induced colon carcinogenesis in mice.
Table 1.
Final body weight, relative organ weights, and colon assessment of mice
| Group | Negative Control | Positive Control | NBT-treated |
|---|---|---|---|
| Treatment | None | AOM/DSS | AOM/DSS/0.05% NBT |
| Body weight (g) | 50.96 ± 1.47 | 48.33 ± 1.22 | 50.52 ± 1.50 |
| Liver weight (mg) | 2348.85 ± 78.36 | 2263.99 ± 94.74 | 2315.85 ± 62.71 |
| Spleen weight (mg) | 238.18 ± 35.58 | 230.67 ± 30.15 | 241.11 ± 38.19 |
| Colon length (mm) | 96.62 ± 2.42a | 87.80 ± 2.90b | 94.13 ± 2.26a |
| Colon weight (mm) | 353.96 ± 35.47 | 357.84 ± 46.07 | 351.10 ± 28.42 |
| Colon W/L ratio (mg/mm) | 3.69 ± 0.29a | 4.00 ± 0.40b | 3.77 ± 0.33a |
| Tumor incidence | 0a | 100%b | 60%c |
| Tumor multiplicity | 0a | 4.97 ± 1.01b | 1.45 ± 0.60c |
Data are shown as the mean ± SD. Different notation indicates statistical significance (p < 0.05, n = 20) by ANOVA.
Figure 1.
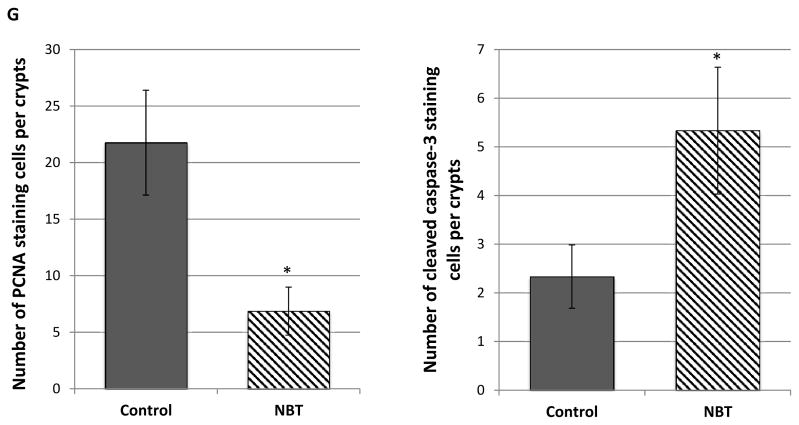
Histological characterization of colonic mucosa and tumors of AOM/DSS-treated mice. (i) H&E staining of colonic tissues of the control (A) and NBT-treated (B) groups (Magnification: 100×); (ii) PCNA staining of colonic mucosa of the control (C) and NBT-treated (D) groups (Magnification: 400×); (iii) Cleaved caspase-3 staining of colonic tumors of the control (E) and NBT-treated (F) groups (Magnification: 400×); (iv) Quantification of PCNA-positive and caspase-3 positive cells in colonic mucosa and tumors (G), * indicates the statistical significance with p < 0.01, n = 6.
3.2 Oral administration of NBT reduced cell proliferation, induced apoptosis, and decreased the levels of pro-inflammatory cytokines in the colon of AOM/DSS-treated mice
Uncontrollable cell proliferation is an important feature of colon carcinogenesis. Proliferating cell nuclear antigen (PCNA) is a cofactor of DNA polymerase δ. The expression of PCNA is increased during G1 phase through S phase of the cell cycle; and it plays a critical role in regulating DNA replication [35]. PCNA therefore is often used as a key marker for cell proliferation [36]. Immunohistochemical analysis showed high number of PCNA positive colonocytes within the colonic mucosal crypts of AOM/DSS-treated control mice (Figure 1C), indicating a high cell proliferation rate. However, dietary treatment with NBT decreased the number of PCNA positive colonocytes by 69% (Figure 1D and 1G). Apoptosis is a major type of programed cell death, and it plays an essential role in balance of death of cancerous cells and survival of normal cells in prevention and treatment of cancer [37]. Induction of apoptosis in cancerous cells is considered an effective strategy for cancer chemoprevention. Caspase-3 is a major regulator of apoptosis, and it can be activated (cleaved) by other initiator caspases such as caspase-9. Cleaved caspase-3 can then further cleave Poly ADP-ribose polymerase (PARP), leading to the loss of DNA repair functions of cancerous cells, and then cell death [38]. As shown in Figure 1E and 1F, colonic tumors from the control group showed low number of cleaved capase-3 positive cells, while higher number of cleaved caspase-3 positive cells (2.3-fold increase, Figure 1G) were observed in the colonic tumors of NBT-treated group. These results demonstrated that dietary treatment with NBT significantly decreased abnormal cell proliferation and induced apoptosis in the colonocytes of AOM/DSS-treated mice, which is believed to contribute to the cancer preventive effects of NBT against colon carcinogenesis.
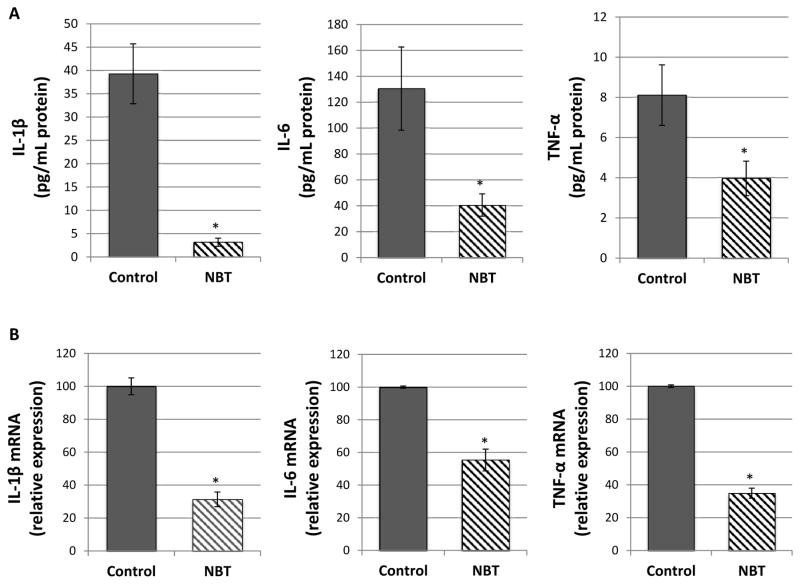
Accumulating studies have demonstrated that improper up-regulation of pro-inflammatory cytokines such as interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α) can accelerate the process of colon carcinogenesis [39, 40]. Using ELISA, we determined the effects of dietary treatment with NBT on the AOM/DSS-induced production of pro-inflammatory cytokines in colonic mucosa. As shown in Figure 2A, NBT treatment resulted in significant decreases in the levels of IL-1β, IL-6 and TNF-α by 92%, 69% and 51%, respectively, compared to those of the control group. Next, we determined the effects of NBT on the mRNA expression levels of pro-inflammatory cytokines by real-time qRT-PCR analysis. As shown in Figure 2B, the mRNA expression levels of IL-1β, IL-6, and TNF-α were significantly reduced by NBT treatment by 69%, 45%, and 65%, respectively, in comparison with those found in the control mice. Together, our results showed that oral administration of NBT inhibited the colon carcinogenesis at least partially by reducing cell proliferation, inducing apoptosis, and suppressing the expression levels of pro-inflammatory cytokines in the colon of AOM/DSS-treated mice.
Figure 2.
Effects of NBT treatment on protein levels (A) and mRNA levels (B) of IL-1β, IL-6, and TNF-α in the colonic mucosa of AOM/DSS-treated mice. Colon tissues samples were randomly collected from the middle and distal colon, and then subjected to ELISA or qRT-PCR analysis. Data are shown as the mean ± SD of three independent experiments. The amount of IL-1β, IL-6, TNF-α mRNA expression was normalized to that of β-actin. * indicates statistically significant differences from the control group (p < 0.01, n = 3).
3.3 Identification and quantification of colonic metabolites of NBT in mice
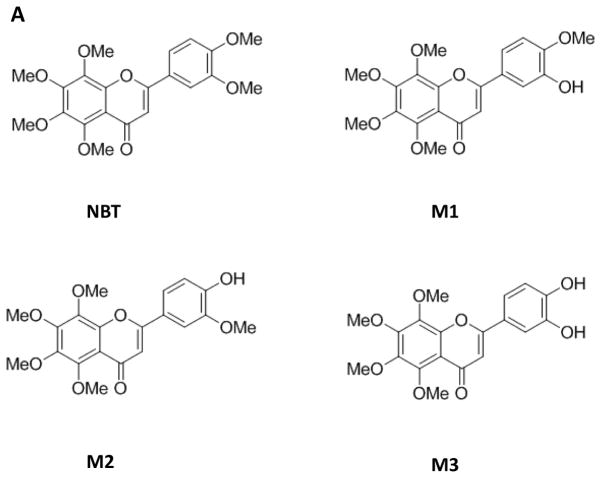
It is well recognized that the in vivo metabolic fate of dietary flavonoids is critical for their biological activities. We and others have reported that orally administered PMFs, such as NBT, tangeretin and 5-demethylnobiletin, undergo extensive biotransformation to produce various metabolites [26–29, 41]. Particularly, three major metabolites were identified in the urine of CD-1 mice after oral administration of NBT [28, 29]. The structure of the three metabolites were shown in Figure 3A, and they are 3′-demethylnobiletin (M1), 4′-demethylnobiletin (M2), and 3′, 4′-didemethylnobiletin (M3), Interestingly, studies have demonstrated that these metabolites of NBT had potent biological activities, including anti-inflammation [29] and anti-atherogenesis [42]. For example, in RAW264.7 macrophages, three metabolites of NBT, especially M2 and M3, showed strong anti-inflammatory effects such as suppressing LPS-induced production of nitric oxide and gene expression of iNOS and COX-2 [29]. Moreover, in THP-1-derived macrophages, M3 showed a strong protective effect against atherogenesis due to its antioxidant activity against LDL oxidation and inhibition on monocyte-to-macrophage differentiation and foam cell formation [42]. Most importantly, the aforementioned beneficial bioactivities of three metabolites were shown to be even stronger than those produced by their parent compound, NBT.
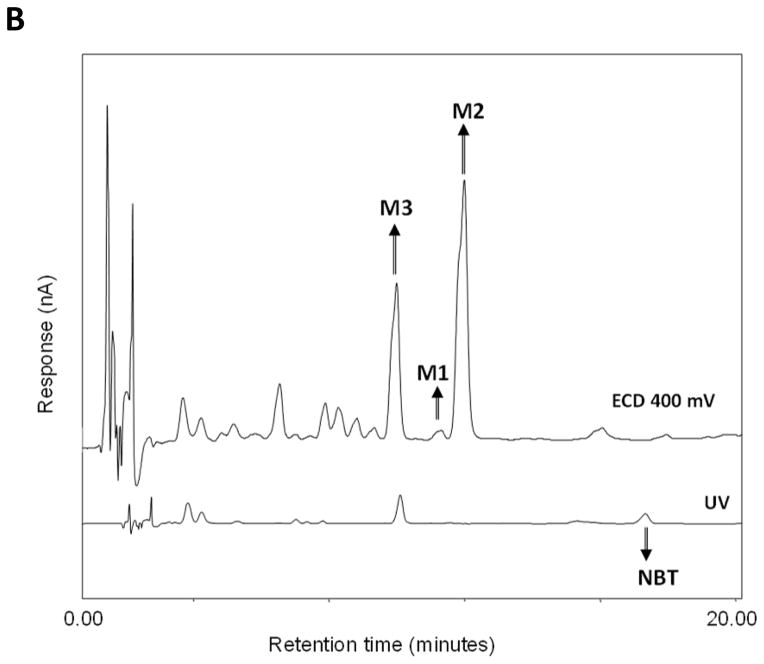
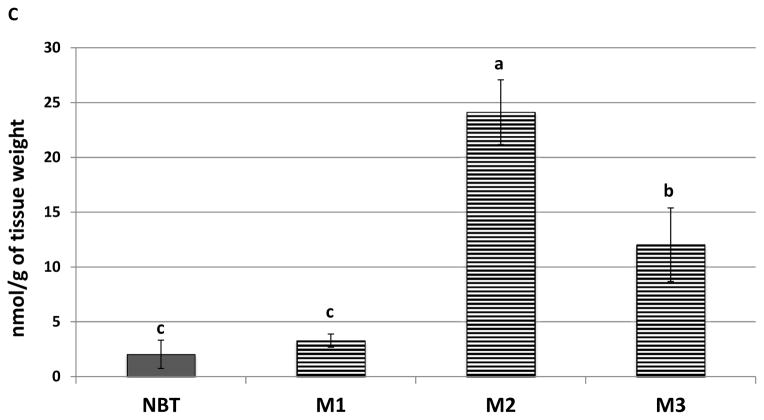
Figure 3.
(A) Chemical structure of NBT and its metabolites M1, M2, and M3. (B)
Representative HPLC chromatogram of NBT, M1, M2, and M3 in the colonic mucosa samples. NBT was detected by UV, and M1, M2, and M3 were detected by electrochemical detection at 400 mv. (C) Quantification of NBT and its metabolites in the colonic mucosa of mice fed with NBT by HPLC analysis. Different notations in the bar charts indicate statistical significance (p < 0.01, n = 6) by ANOVA.
An important goal of this study was to establish the role of biotransformation in the chemopreventive effects of NBT against colitis-associated colon carcinogenesis. In order to do so, the identity and abundance of metabolites of NBT in the colonic mucosa needed to be determined. This is because that metabolites found in the colonic mucosa are likely responsible for the biological activities observed in the colonic mucosa. We hypothesized that the urinary metabolites (M1, M2, and M3) of NBT can also be formed in the colonic mucosa of mice after oral administration of NBT. To confirm this hypothesis, we conducted HPLC analysis on colonic mucosa samples harvested from NBT-fed mice and the control mice [26, 27]. The results showed that the three metabolites M1, M2, and M3 were indeed present in the colonic mucosa of NBT-fed mice (Figure 3B). We chemically synthesized M1, M2, and M3 (purity greater than 98%) and used them as chemical standards for HPLC and mass spectroscopy analysis [26, 27]. It was observed that the colonic metabolites of NBT showed identical retention times and electrochemical responses as M1, M2 and M3 standards. Mass spectroscopy results also confirmed that three colonic metabolites of NBT showed identical molecular weights as M1, M2, and M3, respectively. These results demonstrated that M1, M2, and M3 were the metabolites of NBT in the colonic mucosa of the mice fed with NBT. The exact mechanism for the formation of these colonic metabolites are not known, and it is likely that their formation is the result of phase I & II metabolism as well as biotransformation by gut microbiome.
We further quantified the levels of NBT and its metabolites in the colonic mucosa of NBT-fed mice by HPLC [26, 27]. As shown in Figure 3C, the colonic levels of NBT, M1, M2 and M3 were 2.03, 3.28, 24.13, and 12.03 nmol/g of tissue, respectively. It is noteworthy that the levels of three metabolites M1, M2, and M2 were 1.6-, 11.9- and 5.9-fold higher than that of NBT in the colonic mucosa, respectively, and the level of NBT only accounted for less than 5% of total levels of NBT and three metabolites. This is very useful information because it indicated that colonic metabolites of NBT might play more important roles than NBT itself in the inhibition of colon carcinogenesis after oral administration of NBT, due to their much greater abundance than NBT in the colonic mucosa. Overall, for the first time, we identified M1, M2 and M3 as three major metabolites of NBT found in the colonic mucosa of the mice after long-term oral administration of NBT. Most importantly, we found that the major forms of NBT in the colonic mucosa were its metabolites rather than NBT itself, suggesting the importance of biotransformation in the biological effects of orally administered NBT.
3.4 Colonic metabolites of NBT had stronger inhibitory effects than NBT on the growth of human colon cancer cells
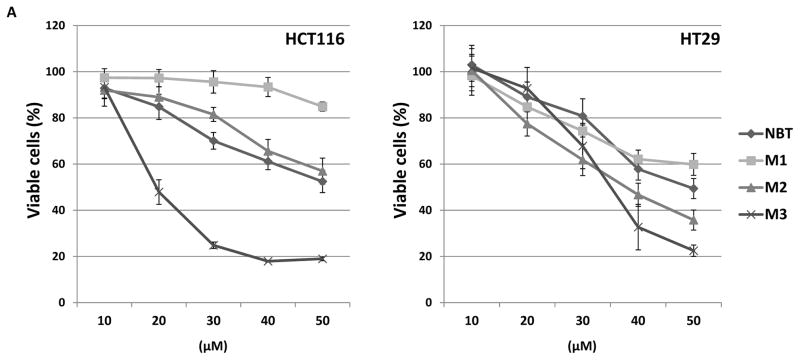
To compare anti-cancer potential of NBT and its colonic metabolites, we determined the inhibitory effects of NBT, M1, M2, and M3 (at a series of concentrations) on the growth of two human colon cancer cell lines, HCT116 and HT29. As shown in Figure 4A, all four compounds showed dose-dependent inhibitory effects on both cell lines after 72 hours of treatment. Overall, M2 and M3 showed stronger inhibitory effects than NBT, especially M3, while M1 showed similar or weaker inhibitory effects than NBT.
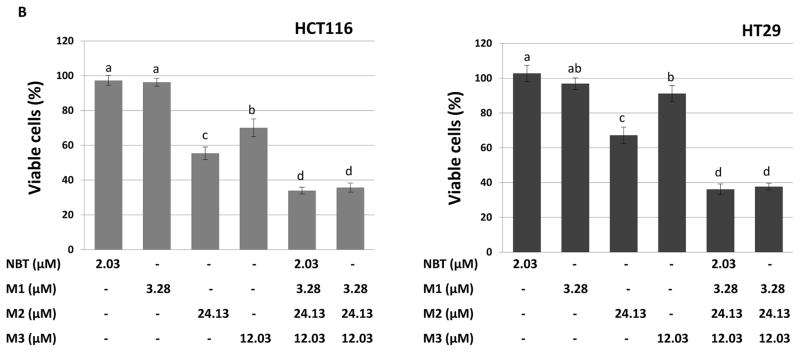
Figure 4.
(A) Growth inhibitory effects of NBT, M1, M2 and M3 on HCT116 and HT29 human colon cancer cells. Cells were seeded in 96-well plates, and after 24 h of incubation, cells were treated with serial concentrations of NBT and its metabolites. After 72 h of incubation, cell viability was measured by MTT assay. Data are shown as mean ± SD (n = 6). (B) Growth inhibitory effects of NBT, M1, M2, M3, NBT+M1+M2+M3, and M1+M2+M3 on HCT116 and HT29 human colon cancer cells. The concentrations of these compounds and their combination were the same as those found in the colonic mucosa of mice fed with NBT (see Figure 3B). Cells were seeded in 96-well plates, and after 24 h of incubation, cells were treated with NBT, its metabolites and their combinations. After 96 h of incubation, cell viability was measured by MTT assay as described. Data are shown as mean ± SD (n = 6). Different notations in the bar charts indicate statistical significance (p < 0.01, n = 6) by ANOVA.
Previously, we studied the inhibitory effects of 5-demethylnobiletin (another important PMF) and its urinary metabolites in human colon cancer cells SW620 and SW480. Our results demonstrated that demethylation at 3′-, 4′-, or both 3′-and 4′-positions of PMF might significantly alter its biological activities [26]. In this study, we found that M3 showed strongest inhibitory effects on human colon cancer cells, indicating that demethylation at both 3′-and 4′-positions enhanced the inhibitory effects of NBT on colon cancer cells. Additionally, M3 has been reported to have stronger inhibitory effects against inflammation and atherogenesis than NBT [29, 42]. It was observed that colonic metabolites M2 and M3 showed stronger inhibitory effects on human colon cancer cells than their parent compound NBT, and the levels of M2 and M3 in the colonic mucosa of NBT-fed mice were about 12- and 6-fold higher than that of NBT. These results suggested that M2 and M3 might have played critical roles in the anti-carcinogenic activities of NBT observed in the NBT-fed mice.
In order to establish the anti-colon cancer effects of NBT and its metabolites in a physiologically relevant manner, we determine their inhibitory effects on colon cancer cells at the concentrations found in the mouse colonic mucosa after oral consumption of NBT (Figure 4B). If we assumed that 1-gram of colonic tissue gave 1 mL of volume, the concentrations of NBT, M1, M2, and M3 in the colonic mucosa were 2.03, 3.28, 24.13, and 12.03 μM, respectively. As shown in Figure 4B, after 96 hours of treatments with the compounds at these concentrations, NBT or M1 did not show any significant inhibition on the HCT116 or HT29 cells. In contrast, M2 caused 45% and 33% inhibition, while M3 caused 30% and 9% inhibition on HCT116 and HT29 cells, respectively. Moreover, the combination of M1, M2, and M3 resulted in 64% and 62% inhibition on HCT116 and HT29 cells, respectively. Most interestingly, the combination of all four compounds (NBT, M1, M2, and M3) did not produce stronger inhibitory effects in comparison with the combination of M1, M2, and M3. Overall, our results demonstrated that colonic metabolites of NBT played more important roles than NBT itself in inhibiting cancer cell growth in the colonic mucosa.
3.5 Colonic metabolites of NBT had stronger effects in inducing cell cycle arrest and apoptosis than NBT
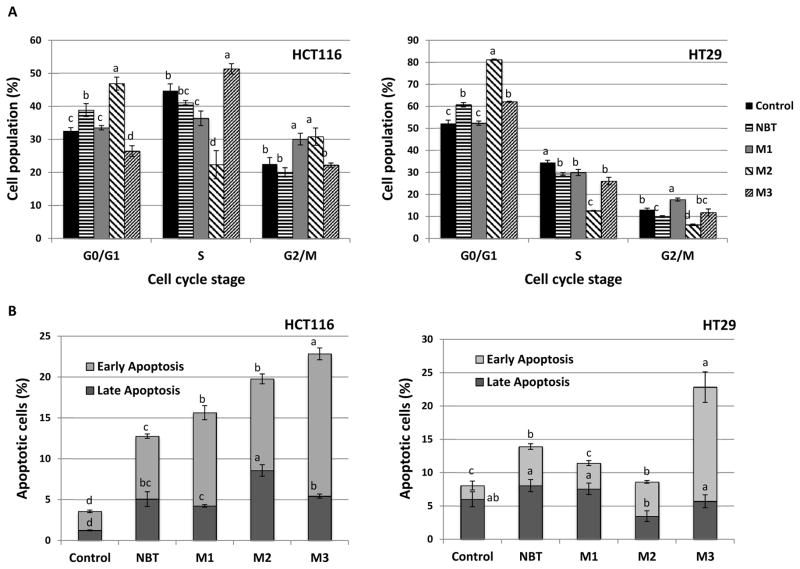
To further characterize the mode of action of NBT and its metabolites in inhibiting the growth of colon cancer cells, we determined their effects on cell cycle and apoptosis by flow-cytometry. As shown in Figure 5A, treatments with NBT (40 μM) or M2 (40 μM) for 24 hours caused cell cycle arrest at G0/G1 phase in both HCT116 and HT29 cells, and the effects of M2 were stronger than those of NBT. In contrast, M1 (40 μM) and M3 (20 μM) led to cell cycle arrest at G2/M and S phases in HCT116 cells, and G2/M and G0/G1 phases in HT29 cells, respectively. Our results showed that different colonic metabolites of NBT had distinct effects on cell cycle progression of human colon cancer cells; suggesting different molecular mechanisms were involved in their actions.
Figure 5.
Effects of NBT (40 μM), M1 (40 μM), M2 (40 μM) and M3 (20 μM) on cell cycle progression (A) and apoptosis (B) of HCT116 and HT29 human colon cancer cells. Cells were seeded in 6-well plates for 24 h, and then treated with NBT and its metabolites. After 24 or 48h of treatments, cells were harvested and subject to cell cycle and apoptosis analysis, respectively Data are shown as mean ± SD. Different notations in the bar charts indicate statistical significance (p < 0.01, n = 3) by ANOVA.
To determine if apoptosis contributed to the growth inhibitory effects of NBT and its metabolites, we performed Annexin-V/Propidium iodine double staining assay to evaluate the cellular apoptosis of HCT116 and HT29 cells. As shown in Figure 5B, after 48 hours of treatment, in comparison to the control cells, both early and late apoptotic cells were significantly increased by all four compounds in HCT116 cells. Treatments with NBT (40 μM), M1 (40 μM) and M2 (40 μM), and M3 (20 μM) increased early apoptotic cell population by 3.3-, 5.0-, 4.9-, and 7.6-fold compared to the control, respectively; while they increased late apoptotic cell population by 4.2-, 3.5-, 7.1-, and 4.5-fold, respectively. Overall, all metabolites showed stronger effects in inducing apoptosis (early plus late) than NBT in HCT116 cells. In HT29 cells, treatments with NBT (40 μM) and M3 (20 μM) induced significant apoptosis with the effects of M3 being stronger than those of NBT. M1 (40 μM) and M2 (40 μM) did not cause significant apoptosis in HT29 cells. These results demonstrated that NBT and its metabolites, especially M3 induced significant apoptosis in human colon cancer cells. It is noteworthy that the structure differences among NBT and its colonic metabolites are the number and position of demethylation, and these differences produced significant distinction in their mode of actions in terms of their effects on cell cycle progression and apoptosis.
3.6 Colonic metabolites of NBT modulated expression of key signaling proteins related with cell cycle and apoptosis regulation
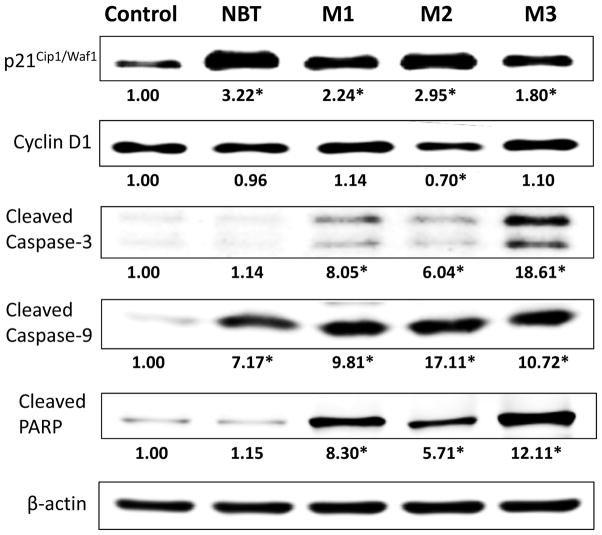
To better understand the molecular mechanisms underlying the anticancer activities of NBT and its colonic metabolites, we analyzed the effects of NBT and its colonic metabolites on key signaling proteins related to cell cycle and apoptosis pathways in HCT116 cells by immunoblotting analysis. Cell cycle-related proteins p21Cip1/Waf1 and cyclin D1 were analyzed after 24 hours of treatment, and apoptosis-related proteins cleaved caspase-3, cleaved caspase-9, and cleaved PARP were analyzed after 48 hours of treatment. As shown in Figure 6, NBT and all three colonic metabolites caused significant increases of p21Cip1/Waf1 expression levels, while only M2 decreased the level of cyclin D1 significantly. The loss of regulated cell cycle and normal cell behavior is one of the important hallmarks of cancer. Cell cycle is tightly controlled by checkpoints, and different cyclin-CDK (cyclin-dependent kinase) complexes. p21Cip1/Waf1 is a key regulator of cell cycle that can inhibit CDK activities and thus control the cell cycle progression at G1 and S phase. The transition of cell cycle from G1 to S phase is mainly regulated by cyclin D-CDK4/6 complexes. Induction of p21Cip1/Waf1 can in turn cause the inactivation or degradation of cyclin D1, leading to cell cycle arrest in G0/G1 stage [43]. Our results illustrated that M2-mediated G0/G1 cell cycle arrest is associated with induction of p21Cip1/Waf1 and reduction of cyclin D1. Although treatments with NBT, M1 and M3 affected the protein levels of p21Cip1/Waf1, they did not significantly inhibit the level of cyclin D1. This may partially explain why NBT, M1, and M3 had weaker or no effects in inducing cell cycle arrest at G0/G1 phase in HCT116 cells in comparison with M2.
Figure 6.
Effects of NBT (40 μM), M1 (40 μM), M2 (40 μM) and M3 (20 μM) on cell cycle and apoptosis related signaling proteins in HCT116 human colon cancer cells. HCT116 cells were seeded in 10-cm dishes for 24 h, and then cells were treated with NBT and its metabolites. After another 24 or 48 h of incubation, cells were collected for Western blotting as described. The numbers underneath of the blots represent band intensity (normalized to β-actin, means of three independent experiments) measured by Image J software. The SDs (all within ±15% of the means) were not shown. β-Actin was served as an equal loading control. * indicates statistical significance in comparison with the control (p < 0.05, n=3).
The evasion of apoptosis is one of the most common cellular abnormalities that may facilitate the development of various cancers [44]. Activation (cleavage) of caspase-9 and its downstream effector caspase-3 play critical roles in triggering cellular apoptosis. Cleavage of caspase-3 can lead to the activation (cleavage) of other key proteins, such as PARP, which can ultimately promote apoptosis by interfering chromatin condensation and DNA fragmentation[45]. Our results showed that colonic metabolites of NBT strongly induced the activation of caspase cascade, including cleavage of caspase-3 and caspase-9, as well as their final protein target PARP in HCT116 cells, while NBT only induced the activation of caspase-9 and had no effect on caspase-3 or PARP (Figure 6). This finding is consistent with previous Annexin-V/PI double staining assay (Figure 5B). Again, our results demonstrated that colonic metabolites of NBT had stronger effects in the activation of caspase cascade for apoptosis in comparison with NBT itself.
5. Concluding remarks
In conclusion, our results demonstrated that oral administration of NBT effectively inhibited colitis-associated colon carcinogenesis in AOM/DSS-treated male CD-1 mice. For the first time, we identified and quantified major metabolites of NBT in the colonic mucosa, namely 3′-demethylnobiletin (M1), 4′-demethylnobiletin (M2), and 3′, 4′-didemethylnobiletin (M3). Moreover, the abundance of these metabolites were about 20-fold higher than that of their parent compound NBT in the colonic mucosa. Our results further demonstrated that colonic metabolites of NBT had stronger anti-cancer effects than NBT, which was evidenced by their superior effects in inhibiting cancer cell growth, inducing cell cycle arrest and apoptosis, and modulating key signaling proteins. Together, our results showed that the chemopreventive effects of orally administered NBT against colon carcinogenesis were closely associated with its colonic metabolites that had potent anti-cancer effects. This study provided a solid scientific basis for using NBT as a chemopreventive agent for colon cancer in human.
Acknowledgments
This work was partially supported by a NIH grant (CA139174), an AICR grant, and a USDA Special Grant on bioactive food components.
Abbreviations
- NBT
nobiletin
- AOM
azoxymethane
- DSS
dextran sulfate sodium
- PMF
polymethoxyflavone
- H&E
haematoxylin and eosin
- PCNA
Proliferating cell nuclear antigen
- IL-1β
interleukin 1β
- IL-6
interleukin 6
- TNF-α
tumor necrosis factor-α
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
Footnotes
X.W., M.S., and H.X. conceived and designed experiments. X.W., M.S., M.W., J.Z., Z.G, and F.X. performed experiments. X.W. and H.X. analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
The authors have declared no conflict of interest.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: how far have we come? Pharmaceutical research. 2010;27:950–961. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Lo CY, Ho CT. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. Journal of agricultural and food chemistry. 2006;54:4176–4185. doi: 10.1021/jf060234n. [DOI] [PubMed] [Google Scholar]
- 5.Murakami A, Nakamura Y, Torikai K, Tanaka T, et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer research. 2000;60:5059–5066. [PubMed] [Google Scholar]
- 6.Yoshigai E, Machida T, Okuyama T, Mori M, et al. Citrus nobiletin suppresses inducible nitric oxide synthase gene expression in interleukin-1beta-treated hepatocytes. Biochemical and biophysical research communications. 2013;439:54–59. doi: 10.1016/j.bbrc.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Xiong Y, Chen D, Yu C, Lv B, et al. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Molecular nutrition & food research. 2015;59:829–842. doi: 10.1002/mnfr.201400614. [DOI] [PubMed] [Google Scholar]
- 8.Minagawa A, Otani Y, Kubota T, Wada N, et al. The citrus flavonoid, nobiletin, inhibits peritoneal dissemination of human gastric carcinoma in SCID mice. Japanese journal of cancer research : Gann. 2001;92:1322–1328. doi: 10.1111/j.1349-7006.2001.tb02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang M, Ogawa K, Asamoto M, Hokaiwado N, et al. Protective effects of citrus nobiletin and auraptene in transgenic rats developing adenocarcinoma of the prostate (TRAP) and human prostate carcinoma cells. Cancer science. 2007;98:471–477. doi: 10.1111/j.1349-7006.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki R, Kohno H, Murakami A, Koshimizu K, et al. Citrus nobiletin inhibits azoxymethane-induced large bowel carcinogenesis in rats. BioFactors. 2004;22:111–114. doi: 10.1002/biof.552210121. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto S, Yasui Y, Tanaka T, Ohigashi H, Murakami A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis. 2008;29:1057–1063. doi: 10.1093/carcin/bgn080. [DOI] [PubMed] [Google Scholar]
- 12.Tang MX, Ogawa K, Asamoto M, Chewonarin T, et al. Effects of nobiletin on PhIP-induced prostate and colon carcinogenesis in F344 rats. Nutrition and cancer. 2011;63:227–233. doi: 10.1080/01635581.2011.523506. [DOI] [PubMed] [Google Scholar]
- 13.Mulvihill EE, Assini JM, Lee JK, Allister EM, et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes. 2011;60:1446–1457. doi: 10.2337/db10-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotito SB, Zhang WJ, Yang CS, Crozier A, Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free radical biology & medicine. 2011;51:454–463. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Cheng C, Yang J, Guo DA. Metabolite profiling and characterization for medicinal herbal remedies. Current drug metabolism. 2012;13:535–557. doi: 10.2174/1389200211209050535. [DOI] [PubMed] [Google Scholar]
- 16.Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. The Journal of nutrition. 2010;140:1363S–1368S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Touil YS, Auzeil N, Boulinguez F, Saighi H, et al. Fisetin disposition and metabolism in mice: Identification of geraldol as an active metabolite. Biochemical pharmacology. 2011;82:1731–1739. doi: 10.1016/j.bcp.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 18.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. The Journal of nutrition. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 19.Oki E, Sakaguchi Y, Toh Y, Oda S, et al. Induction of apoptosis in human tumour xenografts after oral administration of uracil and tegafur to nude mice bearing tumours. British journal of cancer. 1998;78:625–630. doi: 10.1038/bjc.1998.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao H, Hao X, Simi B, Ju J, et al. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis. 2008;29:113–119. doi: 10.1093/carcin/bgm204. [DOI] [PubMed] [Google Scholar]
- 21.Pascal RR. Dysplasia and early carcinoma in inflammatory bowel disease and colorectal adenomas. Human pathology. 1994;25:1160–1171. doi: 10.1016/0046-8177(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Wu WK, Li ZJ, Chan KM, et al. 2,3′,4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits colitis-associated colorectal carcinogenesis in mice. British journal of pharmacology. 2010;160:1352–1361. doi: 10.1111/j.1476-5381.2010.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo S, Qiu P, Xu G, Wu X, et al. Synergistic anti-inflammatory effects of nobiletin and sulforaphane in lipopolysaccharide-stimulated RAW 264. 7 cells. Journal of agricultural and food chemistry. 2012;60:2157–2164. doi: 10.1021/jf300129t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiou YS, Ma NJ, Sang S, Ho CT, et al. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. Journal of agricultural and food chemistry. 2012;60:3441–3451. doi: 10.1021/jf300441p. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Zheng J, Song M, Dong P, Qiu P, et al. Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Molecular nutrition & food research. 2013;57:1999–2007. doi: 10.1002/mnfr.201300211. [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, Bi J, Johnson D, Sun Y, et al. Analysis of 10 Metabolites of Polymethoxyflavones with High Sensitivity by Electrochemical Detection in High-Performance Liquid Chromatography. Journal of agricultural and food chemistry. 2015 doi: 10.1021/jf505545x. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Wang Z, Sang S, Huang MT, Ho CT. Identification of nobiletin metabolites in mouse urine. Molecular nutrition & food research. 2006;50:291–299. doi: 10.1002/mnfr.200500214. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Sang S, Pan MH, Lai CS, et al. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorganic & medicinal chemistry letters. 2007;17:5177–5181. doi: 10.1016/j.bmcl.2007.06.096. [DOI] [PubMed] [Google Scholar]
- 30.Qiu P, Dong P, Guan H, Li S, et al. Inhibitory effects of 5-hydroxy polymethoxyflavones on colon cancer cells. Molecular nutrition & food research. 2010;54(Suppl 2):S244–252. doi: 10.1002/mnfr.200900605. [DOI] [PubMed] [Google Scholar]
- 31.Xiao H, Zhang Q, Lin Y, Reddy BS, Yang CS. Combination of atorvastatin and celecoxib synergistically induces cell cycle arrest and apoptosis in colon cancer cells. Int J Cancer. 2008;122:2115–2124. doi: 10.1002/ijc.23315. [DOI] [PubMed] [Google Scholar]
- 32.Charoensinphon N, Qiu P, Dong P, Zheng J, et al. 5-Demethyltangeretin inhibits human nonsmall cell lung cancer cell growth by inducing G2/M cell cycle arrest and apoptosis. Mol Nutr Food Res. 2013 doi: 10.1002/mnfr.201300136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta pharmacologica Sinica. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 34.De Robertis M, Massi E, Poeta ML, Carotti S, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. Journal of carcinogenesis. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurki P, Vanderlaan M, Dolbeare F, Gray J, Tan EM. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Experimental cell research. 1986;166:209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- 36.McKay JA, Douglas JJ, Ross VG, Curran S, et al. Analysis of key cell-cycle checkpoint proteins in colorectal tumours. The Journal of pathology. 2002;196:386–393. doi: 10.1002/path.1053. [DOI] [PubMed] [Google Scholar]
- 37.Pan MH, Ho CT. Chemopreventive effects of natural dietary compounds on cancer development. Chemical Society reviews. 2008;37:2558–2574. doi: 10.1039/b801558a. [DOI] [PubMed] [Google Scholar]
- 38.Ouyang L, Shi Z, Zhao S, Wang FT, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell proliferation. 2012;45:487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer science. 2004;95:475–480. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114 e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen SE, Breinholt V, Cornett C, Dragsted LO. Biotransformation of the citrus flavone tangeretin in rats. Identification of metabolites with intact flavane nucleus. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2000;38:739–746. doi: 10.1016/s0278-6915(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 42.Lo YH, Pan MH, Li S, Yen JH, et al. Nobiletin metabolite, 3′,4′-dihydroxy-5,6,7,8-tetramethoxyflavone, inhibits LDL oxidation and down-regulates scavenger receptor expression and activity in THP-1 cells. Biochimica et biophysica acta. 2010;1801:114–126. doi: 10.1016/j.bbalip.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Owa T, Yoshino H, Yoshimatsu K, Nagasu T. Cell cycle regulation in the G1 phase: a promising target for the development of new chemotherapeutic anticancer agents. Current medicinal chemistry. 2001;8:1487–1503. doi: 10.2174/0929867013371996. [DOI] [PubMed] [Google Scholar]
- 44.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nature reviews Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 45.Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, et al. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. The Journal of biological chemistry. 1998;273:33533–33539. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]