Abstract
There have been major advances in the diagnosis, staging, risk-stratification, and management of multiple myeloma (MM). In addition to established CRAB (hypercalcemia, renal failure, anemia, and lytic bone lesions) features, new diagnostic criteria include 3 new biomarkers to diagnose the disease: bone marrow clonal plasmacytosis ≥60%, serum involved/uninvolved free light chain ratio ≥100, and >1 focal lesion on magnetic resonance imaging. MM can be classified into several subtypes based on baseline cytogenetics, and prognosis varies according to underlying cytogenetic abnormalities. A Revised International Staging System has been developed which combines markers of tumor burden (albumin, beta-2 microglobulin) with markers of aggressive disease biology (high risk cytogenetics and elevated serum lactate dehydrogenase). Although the approach to therapy remains largely the same, the treatment options at every stage of the disease have changed. Carfilzomib, pomalidomide, and panobinostat have been approved for the treatment of the disease. Elotuzumab, daratumumab, and ixazomib are expected to be approved shortly. These drugs combined with older agents such as cyclophosphamide, dexamethasone, thalidomide, bortezomib, and lenalidomide dramatically increase the repertoire of regimens available for the treatment of MM. This review provides a concise overview of recent advances in MM, including updates to diagnostic criteria, staging, risk-stratification, and management.
INTRODUCTION
The overall survival (OS) of multiple myeloma (MM) has improved significantly in the last 15 years. Among patients with newly diagnosed MM seen at the Mayo Clinic from1971 to 2010, the median overall survival increased from 2.5 years in patients diagnosed prior to 2001, to 4.6 years between the years 2001–2005, and to 6.1 years in patients diagnosed 2006–2010.1,2 Among patients over 65 years, the 6-year OS improved from 31% (2001–2005) to 56% (2006–2010). The Mayo Clinic study includes all patients seen at a single institution including those with poor performance status. OS reported in clinical trials, which almost always exclude patients with serious comorbidities and poor performance status, show even better absolute rates. Survival rates are more striking when one examines patients who are candidates for autologous stem cell transplantation (ASCT); in a recent trial by the Intergroupe Francophone du Myelome (IFN) and the Dana Farber Cancer Institute (DFCI), the 3 year OS rate was 88%.3 These improvements in OS are primarily the result of several new treatment options for newly diagnosed and relapsed MM, most importantly, thalidomide,4 bortezomib,5 and lenalidomide.6,7 Other factors that have contributed to improved survival include early and more accurate diagnosis of MM, advances in supportive care, adjustments to treatment schedules to minimize toxicity, and improved risk-stratification.8,9 It is likely that the outcome of MM patients diagnosed today will surpass the results from even the most recent of studies because several other treatment options are going to be available. In fact, 3 new drugs have been approved for the treatment of MM in the last few years (carfilzomib, pomalidomide, and panobinostat), and 3 others are expected to be approved in the next year (elotuzumab, daratumumab, and ixazomib). Several others show promising single-agent activity and are in various stages of development. This review will provide a concise overview of recent advances in MM, including updates to diagnostic criteria, staging, risk-stratification, and management.
DISEASE DEFINITION
Until recently, MM was defined using strict clinicopathological criteria that required evidence of specific end-organ damage attributable to the underlying clonal plasma cell disorder.10,11 Specifically, hypercalcemia, renal failure, anemia, or bone lesions (CRAB features) felt related to the neoplastic proliferation was needed in order to make a diagnosis of malignancy. In the absence of end-organ damage patients with clonal plasma cell proliferation were considered to have either monoclonal gammopathy of undetermined significance (MGUS) or smoldering multiple myeloma (SMM). SMM carries a much higher risk of progression to malignancy (approximately 10% per year) than MGUS (approximately 1% per year).12,13
The requirement for end-organ damage in order to define a malignancy is unique, and was established decades ago based on the fact that most patients with MGUS and SMM can be asymptomatic and progression free for years without any therapy. Further, treatment options were limited, and the potential for serious toxicity with available treatments (alkylators and steroids) was a major factor in this paradigm. However, this also meant that timely therapy to prevent end-organ damage was not possible, and patients were being observed until evidence of renal failure or bone destruction occurred. In 2014, the International Myeloma Working Group (IMWG) revised the disease definition of MM to enable early diagnosis before end-organ damage occurred.14 This paradigm shift was made possible by 4 key developments in the field. First, several new highly active drugs are now available to treat MM, and these agents have more than doubled the survival of patients with MM.2 Second, specific biomarkers were identified that accurately distinguished patients with SMM who have a high probability (≥80%) or progression to MM within 2 years, thereby providing the opportunity to deliver therapy only to patients with the highest risk, while patients with true MGUS and SMM could continue to be observed.15 Third, advanced imaging modalities, especially low dose whole body computed tomography (CT) and fluoro-deoxyglucose (FDG) positron emission tomography/computed tomographic scans (PET/CT) were shown to detect early bone disease.16 This meant a more accurate initial diagnostic assessment can be done, and in addition patients who are being observed could potentially be diagnosed when bone lesions are still small and non-destructive.17 Finally, a randomized trial in patients with high risk SMM showed a survival advantage to early therapy with lenalidomide and low dose dexamethasone (Rd) which allayed longstanding fears of treating an asymptomatic patient population with cancer chemotherapy.18
New Diagnostic Criteria For MM
The revised IMWG criteria for the diagnosis of MM and related disorders are shown on Table 1.14 The diagnosis of MM requires the presence of one or more myeloma defining events (MDE) in addition to evidence of either 10% or more clonal plasma cells on bone marrow examination or a biopsy-proven plasmacytoma. MDE includes established CRAB features as well as 3 specific biomarkers: clonal bone marrow plasma cells ≥60%, serum free light chain (FLC) ratio ≥100 (provided involved FLC level is ≥100 mg/L), and more than one focal lesion on magnetic resonance imaging (MRI). Each of the new biomarkers are associated with an approximately 80% risk of progression to symptomatic end-organ damage in two or more independent studies.
Table 1.
International Myeloma Working Group Diagnostic Criteria for Multiple Myeloma and Related Plasma Cell Disorders
| Disorder | Disease Definition |
|---|---|
| Non-IgM monoclonal gammopathy of undetermined significance (MGUS) | All 3 criteria must be met:
|
| Smoldering multiple myeloma | Both criteria must be met:
|
| Multiple Myeloma | Both criteria must be met:
|
| IgM Monoclonal gammopathy of undetermined significance (IgM MGUS) | All 3 criteria must be met:
|
| Light Chain MGUS | All criteria must be met:
|
| Solitary Plasmacytoma | All 4 criteria must be met
|
| Solitary Plasmacytoma with minimal marrow involvement** | All 4 criteria must be met
|
Reproduced from Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–e548.
A bone marrow can be deferred in patients with low risk MGUS (IgG type, M protein <15 gm/L, normal free light chain ratio) in whom there are no clinical features concerning for myeloma
Solitary plasmacytoma with 10% or more clonal plasma cells is considered as multiple myeloma
Extreme bone marrow clonal plasmacytosis
Clonal bone marrow plasma cell involvement of ≥60% is rare without concomitant CRAB features. In a Mayo Clinic study, only 6 of 276 patients (2%) had clonal bone marrow plasma cells ≥ 60%19 These patients had rapid progression to symptomatic malignancy with a median progression-free survival (PFS) of 7.7 months.19 In another Mayo Clinic cohort of 651 patients with SMM, only 21 (3.2%) had clonal bone marrow plasma cells ≥ 60%.19 Of these, 95% progressed to MM within 2 years of diagnosis with a median time to progression (TTP) of 7 months. These results were confirmed by the Greek Myeloma Group,20 and by the University of Pennsylvania.21
Marked elevation of serum involved/uninvolved FLC ratio
In SMM, an abnormal involved/uninvolved FLC ratio (≥8) is associated with a higher risk of progression to MM.22 Larsen and colleagues investigated with extreme abnormalities of the serum FLC ratio can be used as a biomarker of malignancy.23 In a study of 586 patients with presumed SMM, an involved/uninvolved FLC ratio ≥100 was seen in 90 patients (15%). The risk of progression to MM within the first 2 years with an FLC ratio ≥100 was 72%; the risk of progression to MM or AL amyloidosis in 2 years was 79%. Kastritis et al studied 96 patients with SMM, and found an involved/uninvolved FLC ratio of ≥100 in 7% of patients; almost all of these patients progressed to MM within 18 months.20 In a third study, at the University of Pennsylvania, SMM patients with an involved/uninvolved FLC ratio ≥100 had a 64% risk of progression within 2 years.21 To reduce possibility of error, in addition to the FLC ratio ≥100, the IMWG also added a requirement for a minimal involved FLC level of at least 100 mg/L in order to be considered as an MDE.14
MRI with more than one focal lesion
In a study by Hillengass et al, 23 of 149 (15%) patients with SMM had more than one focal lesion on whole body MRI.24 The median TTP in these patients was 13 months, and the progression rate at 2 years was 70%. These results were confirmed by Kastritis et al found >1 focal lesion on spinal MRI in 9 of 65 patients (14%) with SMM.25 The median TTP was 15 months and 69% progressed to MM within 2 years. The IMWG added a requirement that focal lesions need to be at least 5mm or more in size, and recommended follow-up examinations in 3–6 months in patients with who had a solitary focal lesion, equivocal findings, or diffuse infiltration.14
Changes to Imaging Requirements for Diagnosis
The updated IMWG criteria clarify that computed tomography (CT), low-dose whole body CT, and positron emission tomography with computerized tomography (PET-CT) can be used to diagnose lytic bone disease in MM.14 These modalities are more sensitive than conventionals whole body skeletal radiographs, and will enable early and accurate diagnosis of MM.16,26,27 In order to qualify as a MDE, one or more sites of osteolytic bone destruction of at least 5 mm or more in size felt secondary to the plasma cell disorder is required. Increased focal or diffuse uptake on PET-CT is alone not adequate for the diagnosis; evidence of actual osteolytic bone destruction on the CT portion of the examination is required. The presence of osteoporosis, vertebral compression fractures, or bone densitometric changes in the absence of lytic lesions is not sufficient evidence of MM bone disease. As with skeletal radiographs, biopsy of one of the bone lesions should be considered if there is any doubt about the diagnosis of MM.
Other Changes to the Diagnostic Criteria
In terms of renal disease, only suspected or proven light chain cast nephropathy is considered as an MDE.14 Other renal disorders associated with M proteins such as light chain deposition disease, membranoproliferative glomerulonephritis, and AL amyloidosis, are considered unique diseases and not MM. An accurate diagnosis of light chain cast nephropathy is essential.28 A renal biopsy to clarify the underlying cause of the renal failure is recommended in patients with suspected cast nephropathy, especially if the serum involved FLC levels are less than 500 mg/L.29
Hyperviscosity, systemic AL amyloidosis, peripheral neuropathy, and recurrent bacterial infections are not considered as MDE.14
NEW DIAGNOSTIC CRITERIA FOR SMM
The revision to the diagnostic criteria for MM also resulted in an updated disease definition for SMM. SMM is now defined by the presence of a serum monoclonal (M) protein of ≥ 3g/dl and/or 10–60% clonal bone marrow plasma cells with no evidence of MDE or amyloidosis (Table 1).14 This definition excludes patients previously considered to have SMM with ultra-high risk of progression (80% within 2 years) who are now classified as MM based on the updated diagnostic criteria. However, this change upstages only a small proportion of patients, and SMM remains a major clinical dilemma with an overall risk of progression of approximately 10% per year for the first 5 years.30 SMM should be distinguished from MGUS, MM and other related plasma cell disorders using the criteria listed on Table 1. At least one advanced imaging exam (PET-CT, low-dose whole body CT, or MRI of the whole body or spine) is recommended in patients with suspected SMM, or solitary plasmacytoma.14,27,31
UPDATED CLASSIFICATION AND RISK-STRATIFICATION OF MM and SMM
In concert with the revisions to the diagnostic criteria for MM and SMM, there have also been revisions to the molecular classification, staging and risk stratification of these disorders. These changes are of importance since they highlight advances in our understanding of disease biology and the effect this has on prognosis and response to therapy.
Molecular Classification
Molecular Cytogenetic Classification of MM
There are several molecular subtypes of MM, associated with several unique differences in disease presentation and prognosis (Table 2).32 For example, trisomic MM appears to respond particularly well to lenalidomide-based therapy,33,34 while t(4;14) MM requires bortezomib-based induction and maintenance for good outcome.35,36 In terms of clinical presentation, t(4;14) MM appears to have a lower predilection for bone disease at diagnosis, while t(14;16) MM is often associated with high levels of serum free light chains (FLC) and a higher risk of acute renal failure at diagnosis.37
Table 2.
Primary Molecular Cytogenetic Classification of Multiple Myeloma
| Subtype | Gene(s)/chromosomes affected* |
Percentage of myeloma patients |
|---|---|---|
| Trisomic MM | Recurrent trisomies involving odd-numbered chromosomes with the exception of chromosomes 1, 13, and 21 |
42 |
| IgH translocated MM | 30 | |
| t(11;14) (q13;q32) | CCND1 (cyclin D1) | 15 |
| t(4;14) (p16;q32) | FGFR-3 and MMSET | 6 |
| t(14;16) (q32;q23) | C-MAF | 4 |
| t(14;20) (q32;q11) | MAFB | <1 |
| Other IgH translocations* |
CCND3 (cyclin D3) in t(6;14) MM |
5 |
|
Combined IgH translocated/trisomic MM |
Presence of trisomies and any one of the recurrent IgH translocations in the same patient |
15 |
| Isolated Monosomy 14 | Few cases may represent 14q32 translocations involving unknown partner chromosomes |
4.5 |
|
Other cytogenetic abnormalities in absence of IgH translocations or trisomy or monosomy 14 |
5.5 | |
| Normal | 3 |
Modified from Kumar S et al. Trisomies in multiple myeloma: impact on survival in patients with high-risk cytogenetics. Blood 2012; 119:2100. © American Society of Hematology.
Includes the t(6;14)(p21;q32) translocation, and rarely, other IgH translocations involving uncommon partner chromosomes
Molecular Cytogenetic Classification of SMM
The initial cytogenetic classification of SMM also has implications for prognosis as shown on Table 3.38,39 Patients with t(4;14) translocation, 17p deletion, and 1q amplification have a higher risk of progression from SMM to MM. Although patients with trisomies are considered to have a better prognosis when diagnosed with MM, they have a higher risk of progression from SMM to MM compared to patients with t(11;14). It is possible that trisomic MM manifests earlier with more obvious bone disease, producing in essence a lead-time bias. Thus the time from SMM to MM is shortened while the time from MM to death appears longer.
Table 3.
Primary Molecular Cytogenetic Classification of Smoldering Multiple Myeloma
| Risk | Cytogenetic Abnormalities | % of patients (N=351) |
Median TTP to Multiple Myeloma (months)a |
Median TTP to Multiple Myeloma or related disorder (months)b |
Median OS from SMM diagnosis (months)c |
Median OS from MM diagnosis (months)d |
|---|---|---|---|---|---|---|
| High-Risk | t(4;14) Del(17p) Gain(1q21)* |
13% | 24 | 24 | 105 | 60 |
|
Intermediate- Risk |
Trisomies | 42% | 34 | 34 | 135 | 77 |
|
Standard- Risk |
Other abnormalities (includes t(11;14), t14;16, t(14;20), combined IgH translocations and trisomies†, and isolated monosomy 13 |
30% | 55 | 54 | 147 | 86 |
| Low-Risk | No abnormalities detected on FISH‡ | 15% | Not reached |
101 | 135 | 112 |
1qamp was not part of this study but included in Table based on data from Neben et al39
Except t(4;14) which is considered high risk with or without concurrent trisomies
Implies adequate probes used to detect del 17p, 1qamp, trisomies, and common IgH translocations
TTP, time to progression; OS, overall survival; SMM, smoldering multiple myeloma; MM, multiple myeloma; IgH, immunoglobulin heavy chain.
P=0.001,
P=0.002,
P=0.12 (global); P=0.02 (high-risk versus standard risk),
P=0.04
Modified from Rajkumar SV et al. Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia. 2013 Aug;27(8):1738-44
Staging and Risk-Stratification
Prognosis in MM is affected by host factors (age, performance status, co-morbidities), disease stage, disease biology, and response to therapy.40,41 Staging of MM has been traditionally done using the Durie-Salmon Staging (DSS)42 or the International Staging System (ISS).43,44 The DSS primarily classified patients based on tumor burden, while the ISS also includes a host factor determinant, namely serum albumin. Neither staging system considers disease biology, a key determinant of overall survival in the disease.
Revised International Staging System for MM
Recently, a Revised International Staging System (RISS) has been adopted by the IMWG.45 The RISS incorporates determinants of disease biology (presence of high risk cytogenetic abnormalities or elevated lactate dehydrogenase level) into the former ISS to create 3 disease stages (Table 4). In a study of 4,445 patients with newly diagnosed MM from 11 international trials, the 5 year survival rate of patients with Stage I, II, and III RISS was 82%, 62%, and 40%, respectively.
Table 4.
Revised International Staging System for Myeloma45
| Stage |
|---|
| Stage 1 All of the following:
|
Stage II
|
| Stage III Both of the following:
|
Risk-Stratification of SMM
With the updated disease definition for MM and SMM, new criteria are also needed to classify patients with SMM into high and low risk groups for monitoring and management. The risk of progression of SMM is approximately 10% per year for the first 5 years; after 5 years, the risk decreases to 3% per year for the next 5 years, and further decreases to approximately 1% per year thereafter.12 Patients with SMM who have a median time to progression (TTP) of 2 years are considered to have high risk SMM (25% per year risk of progression in the first two years)(Table 5).30 Several studies have identified important prognostic markers that can identify such patients.12,22,24,38,39,46–49 Based on encouraging results of a Spanish clinical trial in high risk SMM,18 certain patients with multiple risk factors may even be candidates for MM therapy after a careful consideration of risks and benefits. In contrast, patients with low risk SMM likely have a risk of progression of 5% per year or less, and can be observed.
Table 5.
Criteria for High Risk Smoldering Multiple Myeloma*
| Bone marrow clonal plasma cells ≥10% and any one or more of the following: |
| Serum M protein ≥30g/L |
| IgA SMM |
| Immunoparesis with reduction of two uninvolved immunoglobulin isotypes |
| Serum involved/uninvolved free light chain ratio ≥8 (but less than 100) |
| Progressive increase in M protein level (Evolving type of SMM)† |
| Bone marrow clonal plasma cells 50–60% |
| Abnormal plasma cell immunophenotype (≥95% of bone marrow plasma cells are clonal) and reduction of one or more uninvolved immunoglobulin isotypes |
| t (4;14) or del 17p or 1q gain |
| Increased circulating plasma cells |
| MRI with diffuse abnormalities or 1 focal lesion |
| PET-CT with focal lesion with increased uptake without underlying osteolytic bone destruction |
SMM, smoldering multiple myeloma; M, monoclonal; MRI, magnetic resonance imaging; PET-CT, positron emission tomography-computed tomography
Note that the term smoldering multiple myeloma excludes patients without end-organ damage who meet revised definition of multiple myeloma, namely clonal bone marrow plasma cells ≥60% or serum free light chain (FLC) ratio ≥100 (plus measurable involved FLC level ≥100 mg/L), or more than one focal lesion on magnetic resonance imaging. The risk factors listed in this Table are not meant to be indications for therapy; they are variables associated with a high risk of progression of SMM, and identify patients who need close follow up and consideration for clinical trials
Increase in serum monoclonal protein by ≥25% on two successive evaluations within a 6 month period
Reproduced from: Rajkumar SV, Landgren O, Mateos MV. Smoldering Multiple Myeloma. Blood. 2015 Apr 2. pii: blood-2014-09-568899 © American Society of Hematology.
RECENT ADVANCES IN THE TREAMENT OF MM
Newly Diagnosed MM
The approach to treatment of symptomatic newly diagnosed MM is outlined in Figure 1.10 Typically, patients eligible for ASCT are treated with approximately 4 cycles of induction therapy prior to stem cell harvest. After harvest, patients typically proceed to frontline ASCT. In selected cases (standard risk disease responding well to induction), patients can opt for delayed ASCT; in this setting induction therapy is resumed for 8–12 months, and ASCT is postponed until relapse. Patients who are not candidates for ASCT receive initial therapy for approximately 12–18 months. Upon completion of initial therapy, consideration is given to maintenance therapy as shown in Figure 1.
Figure 1.
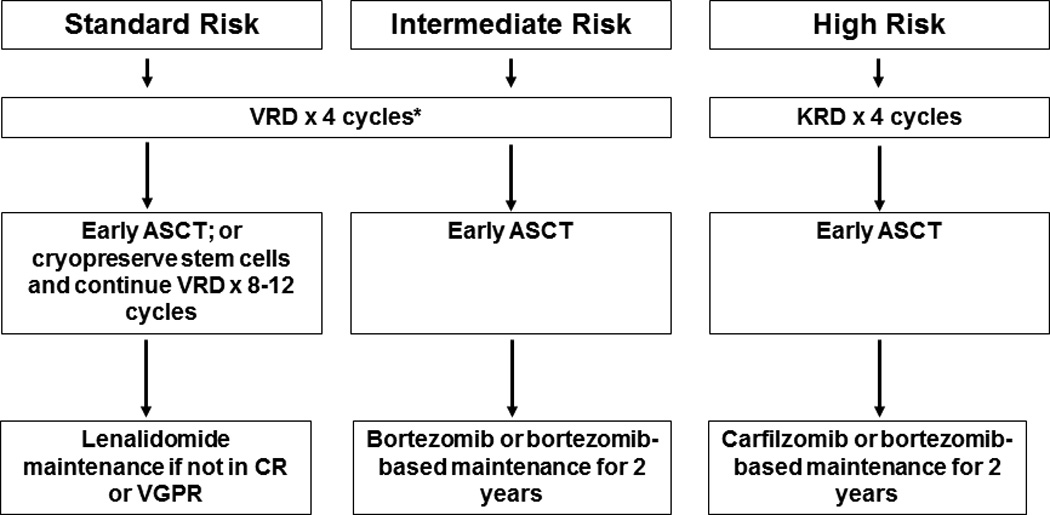
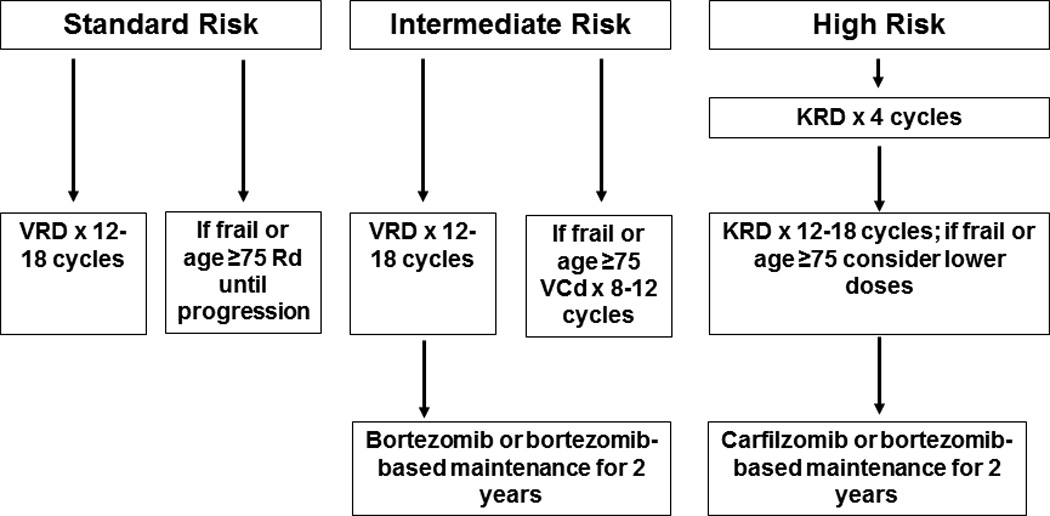
Approach to the treatment of newly diagnosed multiple myeloma in transplant eligible (A) and transplant ineligible (B) patients
Abbreviations: VRD, bortezomib, lenalidomide, dexamethasone; KRD, carfilzomib, lenalidomide, dexamethasone; Rd, lenalidomide plus dexamethasone; VCD, bortezomib, cyclophosphamide, dexamethasone; ASCT, autologous stem cell transplantation; CR, complete response; VGPR, very good partial response
There is a debate concerning the role of achieving a minimal residual disease (MRD) negative state, and pursing MRD negativity as a goal of therapy. Although data show that MRD negative status (as estimated by next generation molecular methods or flow cytometry) has favorable prognostic value, additional trials are needed to determine if changes in treatment need to be made based on MRD status.3,50–52At present, no specific changes in therapy are recommended based on MRD status.
There are many options for initial therapy, and the most common treatment regimens are discussed below. These regimens can also be used at the time of relapse.
Lenalidomide-low dose dexamethasone (Rd)
Rd which combines lenalidomide with a lower dose of dexamethasone (40 mg once weekly) is an active, well-tolerated doublet regimen in newly diagnosed MM.53 It has also become the backbone of many triplet regimens. Stem cell collection with granulocyte stimulating factor (G-CSF) alone may be impaired when Rd is used as induction therapy.54 Thus patients over the age of 65 and those who have received more than 4 cycles of Rd) stem cells must be mobilized with either cyclophosphamide plus G-CSF or with plerixafor.55,56 All patients treated with Rd require anti-thrombosis prophylaxis. Aspirin is adequate for most patients, but in patients who are at higher risk of thrombosis, either low-molecular weight heparin or warfarin is needed.57–59
Bortezomib-containing regimens
Triplet regimens such as bortezomib-thalidomide-dexamethasone (VTD), bortezomib-lenalidomide-dexamethasone (VRD), and bortezomib-cyclophosphamide-dexamethasone (VCD) are highly active in newly diagnosed MM.36,60–63 Recent studies show that a triplet regimen containing an immunomodulatory drug and a proteasome inhibitor offer better response rates, as well as improved progression free survival (PFS) compared with doublets.36,64For example, in randomized trials, VTD has shown better response rates with TD,36 as well as bortezomib plus dexamethasone (VD).64 More importantly, a recent phase III trial has shown that OS is superior with VRD as initial therapy compared with Rd.65 Based on these data VRD or VTD are the preferred regimens for initial therapy in most patients, with the choice between the two options driven mainly by drug-availability. VCD (also referred to as CyBorD) is an alternative; it is less expensive than either VTD or VRD.62,66 However, response rates are lower with VCD compared with VTD. Bortezomib-containing regimens also appear to overcome the poor prognosis associated with the t4;14 translocation, and certain other cytogenetic abnormalities.36,67–69
One of the main adverse effects of bortezomib-containing regimens is peripheral neuropathy. However, the rate of severe neuropathy with bortezomib can be greatly diminished by administering the drug once a week instead of twice-weekly,70,71 and by subcutaneously rather than intravenous administration.72 The once-weekly subcutaneous bortezomib schedule is preferred in all bortezomib-containing regimens except in instances where a rapid response is desirable such as in the treatment of acute renal failure due to cast nephropathy, or spinal cord compression, or plasma cell leukemia. Unlike lenalidomide, bortezomib does not appear to have any adverse effect on stem cell mobilization.73
Carfilzomib-Lenalidomide-Dexamethasone (KRD)
Two phase II trials have reported excellent results with the newly approved proteasome inhibitor carfilzomib when used in combination with lenalidomide and dexamethasone for newly diagnosed MM.74,75 However, more data on safety and efficacy of KRD are needed before this regimen can be recommended in newly diagnosed MM. An exception would be patients with high risk MM in whom it would be reasonable to consider KRD based on the promising phase II studies that suggest higher stringent CR rates than seen historically with VRD. A randomized trial in the United States (referred to as the Endurance trial) is currently ongoing comparing VRD versus KRD as initial therapy.
Multi-drug combinations
Besides the regimens discussed above, another option is multi-agent combination chemotherapy, such as VDT-PACE (bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide).67,68 VDT-PACE is particularly useful in patients with aggressive disease such as plasma cell leukemia or multiple extramedullary plasmacytomas.
Melphalan-based regimens
Melphalan based regimens have fallen out of favor due to concerns about toxicity, impact on stem cell mobilization, secondary myelodysplastic syndrome, and results of a randomized trial showing that outcomes are better with Rd compared to a melphalan-based triplet. In an international phase III trial that compared melphalan, prednisone, thalidomide (MPT) versus Rd for 18 months versus Rd until progression, OS was superior with Rd given until progression compared with MPT.76 This trial provided the first evidence that OS can be improved in patients ineligible for transplant using a regimen that does not contain melphalan. Other melphalan-containing regimens such as bortezomib-melphalan, prednisone (VMP),77,78 bortezomib, thalidomide, prednisone (VTP),70 or melphalan, prednisone, lenalidomide (MPR),79–81 do not offer any major advantage over non-melphalan containing regimens such as VRD or VCD. For example, the VCD regimen can be considered as a minor modification of the VMP regimen, in which cyclophosphamide is used as the alkylating agent in place of melphalan. This variation has the advantage of not affecting stem cell mobilization, and dosing is more predictable. In randomized trials, VTP was not superior to VMP.70 Similarly, randomized trials show that MPR does not improve PFS or OS compared to MP or MPT.79,80 Improved PFS and OS has been reported with a 4-drug regimen of VMPT compared with VMP in a randomized phase III trial.82 However, patients in the VMPT arm received maintenance therapy with bortezomib and thalidomide, while patients in the VMP arm did not receive any additional therapy beyond 9 months making it difficult to determine if the OS difference is due to the addition of the fourth drug to the induction regimen or to the addition of maintenance. Additional data and longer follow up are needed. Overall, melphalan-based regimens are recommended in the frontline setting only if there is lack of access to VRD, VTD, or VCD.
Hematopoietic Stem Cell Transplantation
Autologous stem cell transplantation (ASCT)
ASCT improves median OS in MM by approximately 12 months.83–86 Hence it should be considered in all eligible patients. For most patients, early ASCT after 4 cycles of initial therapy is preferred. However, randomized trials show that OS is similar whether ASCT is done early (immediately following 4 cycles of induction therapy) or delayed (at the time of relapse as salvage therapy).87–89 The most recent of these trials was conducted in the context of modern therapy with VRD and lenalidomide maintenance. Although an improvement in PFS was apparent, no difference in OS has yet emerged.3 Based on these results, a delayed approach to ASCT can be considered in selected patients (Figure 1) with standard risk MM who respond well to initial therapy. Although two randomized trials found a survival advantage with tandem (double) ASCT compared with single ASCT, the benefit primarily seen in patients failing to achieve a complete response (CR) or very good partial response (VGPR) with the first ASCT.90,91 Two other randomized trials did not find such a benefit, and the role of tandem ASCT in the context of modern therapy is unclear.92,93
Allogeneic Transplantation
Allogeneic stem cell transplantation is not recommended as part of initial therapy. There are conflicting data about clinical benefit, and the treatment related mortality (TRM) rate (10–20%) remains a concern. In patients who live beyond the first year, high graft versus host disease (GVHD) rates even with non-myeloablative allogeneic transplantation, and likelihood of relapse remain formidable issues.94 It would be reasonable to consider allogenic transplantation in selected young patients with high risk disease who are willing to accept a high TRM and the unproven nature of this therapy for a chance at better long-term survival.
Post-transplant consolidation/maintenance therapy
In general, the term “consolidation” refers to a short course of therapy following definitive initial therapy, while “maintenance” refers to a more prolonged course of treatment with a lower intensity regimen. In MM studies regarding the value of consolidation per se in the context of uniform maintenance therapy are limited. The role of maintenance has been addressed by several trials over the years, but most provided disappointing results. Thalidomide has shown modest PFS and OS benefit as maintenance therapy in two randomized trials, but is limited by significant non-hematologic toxicity.95,96 Two randomized trials have shown better PFS with lenalidomide as post ASCT maintenance therapy.97,98 But there is an increased risk of second cancers with lenalidomide maintenance in both trials, and it is not clear if patients in the control arm of these trials had uniform access to lenalidomide at relapse. The benefit of lenalidomide maintenance seems to be restricted to patients who received lenalidomide as induction therapy.99 In patients with high and intermediate risk MM, bortezomib administered every other week may be a better strategy for maintenance.35
Relapsed Multiple Myeloma
Almost all patients with MM eventually relapse. The remission duration in relapsed MM decreases with each regimen.100 The median PFS and OS in patients with relapsed MM refractory to lenalidomide and bortezomib is poor, with median times of 5 months and 9 months, respectively.101 The most commonly used options for the treatment of relapsed MM are the same regimens discussed under the treatment of newly diagnosed MM.102,103 There are several key principles to consider. First, if relapse occurs off therapy, several months or years more after stopping therapy, it is reasonable to re-administer the same regimen that was initially effective. Second, if patients are eligible for ASCT, and have either had an excellent outcome with a prior ASCT or have never had an ASCT, it is important to consider transplantation as an early salvage option. Third, the aggressiveness of the regimen chosen is proportional to the aggressiveness of the relapse; thus in elderly patients with indolent paraprotein only relapse it is reasonable to use a double like pomalidomide plus low dose dexamethasone (Pd). Finally, patients with relapsed MM should always be considered for enrollment on to clinical trials. Major regimens used in the treatment of MM, including relapsed disease are listed in Table 6.36,53,60–62,104–107 Recent advances in the treatment of relapsed MM, including new active agents and results of major randomized trials are discussed below (Table 7).108–113
Table 6.
Major Treatment Regimens in Multiple Myeloma
| Regimen | Usual Dosing Schedule* |
|---|---|
| Doublets | |
| Lenalidomide-Dexamethasone (Rd)53 | Lenalidomide 25 mg oral days 1–21 every 28 days Dexamethasone 40 mg oral days 1, 8, 15, 22 every 28 days Repeated every 4 weeks |
| Pomalidomide-Dexamethasone (Pom/Dex)104 | Pomalidomide 4 mg days 1–21 Dexamethasone 40 mg orally on days on days 1, 8, 15, 22 Repeated every 4 weeks |
| Triplets | |
| Bortezomib-Thalidomide- Dexamethasone (VTD)**36 |
Bortezomib 1.3 mg/m2 intravenous days 1, 8, 15, 22 Thalidomide 100–200 mg oral days 1–21 Dexamethasone 20 mg on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 4 weeks × 4 cycles as pre-transplant induction therapy |
| Bortezomib- Cyclophosphamide- Dexamethasone** (VCD or CyBorD)60,62 |
Cyclophosphamide 300 mg/m2 orally on days 1, 8, 15 and 22 Bortezomib 1.3 mg/m2 intravenously on days 1, 8, 15, 22 Dexamethasone 40 mg orally on days on days 1, 8, 15, 22 Repeated every 4 weeks† |
| Bortezomib-Lenalidomide- Dexamethasone (VRD)**60,61 |
Bortezomib 1.3 mg/m2 intravenous days 1, 8, 15 Lenalidomide 25 mg oral days 1–14 Dexamethasone 20 mg on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 3 weeks‡ |
| Carfilzomib- Cyclophosphamide- Dexamethasone (CCyD) ‡‡ 105 |
Carfilzomib 20 mg/m2 (Cycle 1) and 36 mg/ m2 (subsequent cycles) intravenously on days 1, 2, 8, 9, 15, 16 Cyclophosphamide 300 mg/m2 orally on days 1, 8, 15 Dexamethasone 40 mg orally on days on days 1, 8, 15 Repeated every 4 weeks† |
| Carfilzomib-Lenalidomide- Dexamethasone (KRD)106 |
Carfilzomib 27 mg/ m2 intravenously on days 1, 2, 8, 9, 15, 16 (Note: Cycle 1, day 1 and 2 carfilzomib dose is 20 mg/m2) Lenalidomide 25 mg oral days 1–21 Dexamethasone 20 mg on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 4 weeks |
| Carfilzomib-Pomalidomide- Dexamethasone 107 |
Carfilzomib 27 mg/ m2 intravenously on days 1, 2, 8, 9, 15, 16 (Note: Cycle 1, day 1 and 2 carfilzomib dose is 20 mg/m2) Pomalidomide 4 mg oral days 1–21 Dexamethasone 40 mg days 1, 8, 15, 22 Repeated every 4 weeks |
All doses need to be adjusted for performance status, renal function, blood counts, and other toxicities
Doses of dexamethasone and/or bortezomib reduced based on subsequent data showing lower toxicity and similar efficacy with reduced doses.
The day 22 dose of all 3 drugs is omitted if counts are low, or after initial response to improve tolerability, or when the regimen is used as maintenance therapy; When used as maintenance therapy for high risk patients, further delays can be instituted between cycles.
Omit day 15 dose if counts are low or when the regimen is used as maintenance therapy; When used as maintenance therapy for high risk patients, lenalidomide dose may be decreased to 10–15 mg per day, and delays can be instituted between cycles as done in total therapy protocols.67,68
Dosing based on trial in newly diagnosed patients; in relapsed patients cycle 2 Carfilzomib dose is 27 mg/m2 consistent with approval summary Modified from Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol 2014;89:998–1009.
Table 7.
Results of Recent Randomized Studies in Relapsed Myeloma
| Trial | Regimen | No. of patients |
Overall response rate (%) |
CR (%) | Progression- free survival (Median in months) |
P value for progression free survival |
Overall survival* |
P value for overall survival |
|---|---|---|---|---|---|---|---|---|
| San Miguel et al108 | Pd | 302 | 31 | 1 | 4.0 | <0.0001 | 12.7 (median in months) |
|
| Dex | 153 | 10 | 0 | 1.9 | 8 (median in months) |
|||
| Stewart et al109 | KRd | 396 | 87 | 32 | 26.3 | 0.0001 | 2-year survival 73.3% |
0.04 |
| Rd | 396 | 67 | 14 | 17.6 | 2-year survival 65% |
|||
| Dimopoulos et al110 | Kd | 464 | 77 | 13 | 18.7 | <0.0001 | 2-year survival 65% |
0.06 |
| Vd | 465 | 63 | 6 | 9.4 | 2-year survival 72% |
|||
| San Miguel et al111 | Pano-Vd | 387 | 61 | 11 | 12 | <0.0001 | 33.7 (median in months) |
0.26 |
| Vd | 381 | 55 | 6 | 8.1 | 30.4 (median in months) |
|||
| Lonial et al112 | Elo-Rd | 321 | 79 | 4 | 19.4 | <0.001 | N/A | N/A |
| Rd | 325 | 66 | 7 | 14.9 | N/A | |||
| Moreau et al113 | IRd | |||||||
| Rd |
Estimated from survival curves when not reported
Abbreviations: Pd, pomalidomide, dexamethasone; Dex, high dose dexamethasone; KRd, carfilzomib, lenalidomide, dexamethasone; Rd, lenalidomide plus dexamethasone; Kd, carfilzomib, dexamethasone; Vd, bortezomib, dexamethasone; Pano-Vd, Panobinostat, bortezomib, dexamethasone; Elo-Rd, Elotuzumab, lenalidomide, dexamethasone; IRd, ixazomib, lenalidomide, dexamethasone; N/A, not available; CR, complete response.
Pomalidomide
Pomalidomide is an analog of lenalidomide and thalidomide recently approved for the treatment of relapsed refractory MM. It has significant activity in relapsed refractory MM, even in patients failing lenalidomide.114,115 Response rate in patients who are dual-refractory (refractory to both lenalidomide and bortezomib) is approximately 30%.104,116 In a randomized trial (n=302), Pd was found superior to high-dose dexamethasone in patients refractory to other forms of therapy for MM; median PFS 4.0 months versus 1.9 months, respectively, P<0.0001)(Table 7).108 Several pomalidomide-containing combinations have been developed, and few selected regimens are listed in Table 6.
Carfilzomib
Carfilzomib is a novel keto-epoxide tetrapeptide proteasome inhibitor approved for the treatment of relapsed MM. In a phase 2 study (PX-171-003-A1), 266 patients were treated with single-agent carfilzomib, including 80% of patients who were refractory or intolerant to both bortezomib and lenalidomide.117 The overall response rate was 24%, and the median duration of response was 7.8 months. The most common side effects were fatigue (49%), anemia (46%), nausea (45%), and thrombocytopenia (39%).117 Neuropathy was minimal. In a separate phase II trial (PX-171-004) that treated 129 patients who were bortezomib naïve, the response rate with single-agent carfilzomib was approximately 50%.118 Carfilzomib has been combined with other active agents, and common regimens that can be used in relapsed refractory patients are listed in Table 6.105,106 In a phase III trial of 792 patients, KRD was associated with better response rates, PFS, and OS compared with Rd (Table 7).109 PFS was 26.3 months with KRD versus 17.6 months in the control group; P=0.0001. The 2-year survival rates were 73.3% and 65.0%, respectively, P=0.04. Based on these results, KRD is now an important option for the treatment of relapsed MM. In another randomized trial, carfilzomib/dexamethasone demonstrated a doubling of PFS compared with bortezomib/dexamethasone in relapsed MM; PFS 18.7 months versus 9.4 months, respectively, P<0.001(Table 7).110 However, the dose of carfilzomib used in this trial (56mg/m2) is twice the approved dose, and dose of bortezomib was suboptimal (twice-weekly schedule). More data are needed before concluding that carfilzomib is preferred as an earlier option at relapse than bortezomib, especially since bortezomib is more convenient and less expensive. Carfilzomib has a lower risk of neurotoxicity than bortezomib, but a small proportion (5%) of patients may experience serious cardiac side effects. Several carfilzomib-containing combinations have been developed, and few selected regimens are listed in Table 6.
Panobinostat
Panobinostat is a pan-deacetylase inhibitor approved for the treatment of relapsed and refractory MM.111 It is believed to block the aggresome pathway, which functions as an alternative protein degradation pathway and serves as a mechanism of resistance to bortezomib and other proteasome inhibitors.119,120 In a randomized trial of 768 patients, bortezomib/dexamethasone plus panobinostat had longer PFS compared with bortezomib/dexamethasone plus placebo; median PFS 12 months versus 8.1 months, respectively, P<0·0001(Table 7).111 The main side effects are grade 3 diarrhea in approximately 25% of patients.
Elotuzumab
Elotuzumab is a monoclonal antibody targeting the signaling lymphocytic activation molecule F7 (SLAMF7). It does not have single agent activity, but had higher than expected responses when combined with Rd in phase II trials.112 In a phase III trial of 646 patients, PFS was longer with elotuzumab plus Rd versus Rd, median PFS 19.4 months versus 14.9 months, respectively, P<0.001(Table 7).112 Elotuzumab is also well tolerated, and is expected to be approved in the United States within the next few months.
Anti-CD38 monoclonal antibodies
Two monoclonal antibodies (daratumumab and SAR650984) targeting CD38 have shown promise in relapsed, refractory MM. In a phase II trial, daratumumab as a single-agent was produced a response rate of approximately 30% in heavily pre-treated patients.121 These are very encouraging results and it is expected that daratumumab will be approved in the United States for use in relapsed refractory MM based on these data. SAR650984 has also shown single-agent activity in relapsed MM.
Ixazomib
Ixazomib is an oral proteasome inhibitor that is active in both the relapsed refractory setting and in newly diagnosed MM. In a randomized controlled trial in relapsed MM, ixazomib, lenalidomide, dexamethasone (IRd) was found to improve PFS compared with Rd (Table 7).113 Based on these results it is anticipated that ixazomib will secure regulatory approval soon. It has the advantage of once-weekly oral administration. Compared with bortezomib it has more gastrointestinal adverse events, but lower risk of neurotoxicity.
Other Emerging Options
Other promising agents being tested in relapsed MM which have demonstrated single-agent activity include marizomib, a new proteasome inhibitor, oprozomib, an oral proteasome inhibitor related to carfilzomib; filanesib, a kinesin spindle protein inhibitor; dinaciclib, a cyclin dependent kinase inhibitor; ABT-199, a selective BCL-2 inhibitor, and LGH-447, pan PIM kinase inhibitor.
Acknowledgements
Supported in part by grants CA 107476 and CA 168762 from the National Cancer Institute, Rockville, MD, USA.
Footnotes
Disclosure of Conflicts of Interest
SVR declares no conflict of interest.
Authorship Contribution Statement
SVR conceived of the paper, researched the literature, and wrote the manuscript.
REFERENCES
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2013 doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attal Michel, Lauwers-Cances Valerie, Hulin Cyrille, et al. Autologous Transplantation for Multiple Myeloma in the Era of New Drugs: A Phase III Study of the Intergroupe Francophone Du Myelome (IFM/DFCI 2009 Trial). ASH Annual Meeting Abstracts.2015. [Google Scholar]
- 4.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma [see comments] N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma.[see comment] N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma 10.1182/blood-2006-04-015909. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan GJ, Davies FE, Gregory WM, et al. First-line and Ongoing Treatment with Zoledronic Acid Improves Overall Survival in Patients with Multiple Myeloma: Results of the MRC IX Randomised Controlled Trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikhael JR, Dingli D, Roy V, et al. Management of Newly Diagnosed Symptomatic Multiple Myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines 2013. Mayo Clin Proc. 2013;88:360–376. doi: 10.1016/j.mayocp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV. Treatment of Multiple Myeloma. Nature Rev Clin Oncol. 2011;8:479–491. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajkumar SV, Gahrton G, Bergsagel PL. Approach to the treatment of multiple myeloma: a clash of philosophies. Blood. 2011;118:3205–3211. doi: 10.1182/blood-2011-06-297853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyle RA, Remstein ED, Therneau TM, et al. Clinical Course and Prognosis of Smoldering (Asymptomatic) Multiple Myeloma. N Engl J Med. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 13.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 15.Rajkumar SV, Merlini G, San Miguel JF. Redefining myeloma. Nature Rev Clin Oncol. 2012;9:494–496. doi: 10.1038/nrclinonc.2012.128. [DOI] [PubMed] [Google Scholar]
- 16.Zamagni E, Nanni C, Patriarca F, et al. A prospective comparison of 18F–fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92:50–55. doi: 10.3324/haematol.10554. [DOI] [PubMed] [Google Scholar]
- 17.Regelink JC, Minnema MC, Terpos E, et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. British journal of haematology. 2013;162:50–61. doi: 10.1111/bjh.12346. [DOI] [PubMed] [Google Scholar]
- 18.Mateos M-V, Hernández M-T, Giraldo P, et al. Lenalidomide plus Dexamethasone for High-Risk Smoldering Multiple Myeloma. N Engl J Med. 2013;369:438–447. doi: 10.1056/NEJMoa1300439. [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Larson D, Kyle RA. Diagnosis of smoldering multiple myeloma. The New England journal of medicine. 2011;365:474–475. doi: 10.1056/NEJMc1106428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastritis E, Terpos E, Moulopoulos L, et al. Extensive bone marrow infiltration and abnormal free light chain ratio identifies patients with asymptomatic myeloma at high risk for progression to symptomatic disease. Leukemia. 2012;27:947–953. doi: 10.1038/leu.2012.309. [DOI] [PubMed] [Google Scholar]
- 21.Waxman AJ, Mick R, Garfall AL, et al. Modeling the risk of progression in smoldering multiple myeloma. J Clin Oncol. 2014;32:A8607. [Google Scholar]
- 22.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785–789. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen JT, Kumar SK, Dispenzieri A, Kyle RA, Katzmann JA, Rajkumar SV. Serum free light chain ratio as a biomarker for high-risk smoldering multiple myeloma. Leukemia. 2013;27:941–946. doi: 10.1038/leu.2012.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillengass J, Fechtner K, Weber MA, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1606–1610. doi: 10.1200/JCO.2009.25.5356. [DOI] [PubMed] [Google Scholar]
- 25.Kastritis E, Moulopoulos LA, Terpos E, Koutoulidis V, Dimopoulos MA. The prognostic importance of the presence of more than one focal lesion in spine MRI of patients with asymptomatic (smoldering) multiple myeloma. Leukemia. 2014 doi: 10.1038/leu.2014.230. [DOI] [PubMed] [Google Scholar]
- 26.Bartel TB, Haessler J, Brown TL, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–2076. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siontis B, Kumar S, Dispenzieri A, et al. Positron emission tomography-computed tomography in the diagnostic evaluation of smoldering multiple myeloma: identification of patients needing therapy. Blood Cancer J. 2015;5:e364. doi: 10.1038/bcj.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonsalves WI, Leung N, Rajkumar SV, et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2015;5:e296. doi: 10.1038/bcj.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchison CA, Batuman V, Behrens J, et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2012;8:43–51. doi: 10.1038/nrneph.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015;125:3069–3075. doi: 10.1182/blood-2014-09-568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimopoulos MA, Hillengass J, Usmani S, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:657–664. doi: 10.1200/JCO.2014.57.9961. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Fonseca R, Ketterling RP, et al. Trisomies in multiple myeloma: impact on survival in patients with high-risk cytogenetics. Blood. 2012;119:2100–2105. doi: 10.1182/blood-2011-11-390658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandey S, Rajkumar SV, Kapoor P, et al. Impact Of FISH Abnormalities On Response To Lenalidomide In Patients With Multiple Myeloma. 2013 [Google Scholar]
- 34.Vu T, Gonsalves W, Kumar S, et al. Characteristics of exceptional responders to lenalidomide-based therapy in multiple myeloma. Blood Cancer J. 2015;5:e363. doi: 10.1038/bcj.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonneveld P, Schmidt-Wolf IGH, van der Holt B, et al. Bortezomib Induction and Maintenance Treatment in Patients With Newly Diagnosed Multiple Myeloma: Results of the Randomized Phase III HOVON-65/ GMMG-HD4 Trial. Journal of Clinical Oncology. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 36.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg AJ, Rajkumar SV, Therneau TM, Singh PP, Dispenzieri A, Kumar SK. Relationship between initial clinical presentation and the molecular cytogenetic classification of myeloma. Leukemia. 2014;28:398–403. doi: 10.1038/leu.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajkumar SV, Gupta V, Fonseca R, et al. Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia. 2013;27:1738–1744. doi: 10.1038/leu.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neben K, Jauch A, Hielscher T, et al. Progression in smoldering myeloma is independently determined by the chromosomal abnormalities del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4325–4332. doi: 10.1200/JCO.2012.48.4923. [DOI] [PubMed] [Google Scholar]
- 40.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell SJ, Rajkumar SV. Multiple myeloma and the road to personalised medicine. Lancet Oncol. 2011;12:617–619. doi: 10.1016/S1470-2045(11)70143-7. [DOI] [PubMed] [Google Scholar]
- 42.Durie BG, Salmon SE. A clinical staging system for multiple myeloma Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 43.Greipp PR, San Miguel JF, Durie BG, et al. International Staging System for Multiple Myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 44.Hari PN, Zhang MJ, Roy V, et al. Is the international staging system superior to the Durie-Salmon staging system? A comparison in multiple myeloma patients undergoing autologous transplant. Leukemia. 2009;23:1528–1534. doi: 10.1038/leu.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez-Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–2592. doi: 10.1182/blood-2007-05-088443. [DOI] [PubMed] [Google Scholar]
- 47.Rosinol L, Blade J, Esteve J, et al. Smoldering multiple myeloma: natural history and recognition of an evolving type. British journal of haematology. 2003;123:631–636. doi: 10.1046/j.1365-2141.2003.04654.x. [DOI] [PubMed] [Google Scholar]
- 48.Dhodapkar MV, Sexton R, Waheed S, et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120) Blood. 2014;123:78–85. doi: 10.1182/blood-2013-07-515239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bianchi G, Kyle RA, Larson DR, et al. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia. 2013;27:680–685. doi: 10.1038/leu.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125:3059–3068. doi: 10.1182/blood-2014-11-568907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123:3073–3079. doi: 10.1182/blood-2014-01-550020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paiva B, Chandia M, Puig N, et al. The prognostic value of multiparameter flow cytometry minimal residual disease assessment in relapsed multiple myeloma. Haematologica. 2015;100:e53–e55. doi: 10.3324/haematol.2014.115162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 55.Kumar S, Giralt S, Stadtmauer EA, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114:1729–1735. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]
- 56.Giralt S, Stadtmauer EA, Harousseau JL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100) Leukemia. 2009 doi: 10.1038/leu.2009.127. [DOI] [PubMed] [Google Scholar]
- 57.Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011;29:986–993. doi: 10.1200/JCO.2010.31.6844. [DOI] [PubMed] [Google Scholar]
- 58.Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for newly-diagnosed multiple myeloma patients treated with lenalidomide. Blood. 2011 doi: 10.1182/blood-2011-03-344333. [DOI] [PubMed] [Google Scholar]
- 59.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–423. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 60.Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–4382. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 61.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337–1341. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reeder CB, Reece DE, Kukreti V, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010;115:3416–3417. doi: 10.1182/blood-2010-02-271676. [DOI] [PubMed] [Google Scholar]
- 64.Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752–5758. doi: 10.1182/blood-2011-05-355081. [DOI] [PubMed] [Google Scholar]
- 65.Durie Brian GM, Hoering Antje, Rajkumar S Vincent, et al. Bortezomib, Lenalidomide and Dexamethasone vs. Lenalidomide and Dexamethasone in Patients (Pts) with Previously Untreated Multiple Myeloma Without an Intent for Immediate Autologous Stem Cell Transplant (ASCT): Results of the Randomized Phase III Trial SWOG S0777. ASH Annual Meeting Abstracts; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar SK, Ma E, Engebretson AE, et al. Treatment outcomes, health-care resource utilization and costs of bortezomib and dexamethasone, with cyclophosphamide or lenalidomide, in newly diagnosed multiple myeloma. Leukemia. 2015 doi: 10.1038/leu.2015.225. [DOI] [PubMed] [Google Scholar]
- 67.Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. British Journal of Haematology. 2007;138:176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 68.van Rhee F, Szymonifka J, Anaissie E, et al. Total therapy 3 for multiple myeloma: prognostic implications of cumulative dosing and premature discontinuation of VTD maintenance components, bortezomib, thalidomide and dexamethasone, relevant to all phases of therapy. Blood. 116:1220–1227. doi: 10.1182/blood-2010-01-264333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nair B, van Rhee F, Shaughnessy JD, Jr, et al. Superior results of Total Therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with VRD maintenance. Blood. 2010;115:4168–4173. doi: 10.1182/blood-2009-11-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mateos MV, Oriol A, Martinez-Lopez J. Bortezomib/melphalan/prednisone (VMP) versus Bortezomib/Thalidomide/Prednisone (VTP) as induction therapy followed by maintenance treatment with Bortezomib/Thalidomide (VT) versus Bortezomib/Prednisone (VP): A randomised trial in elderly untreated patients with multiple myeloma older than 65 years. Lancet Oncol. 2010;11:934–941. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 71.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28:5101–5109. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 72.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 73.Moreau P, Hulin C, Marit G, et al. Stem cell collection in patients with de novo multiple myeloma treated with the combination of bortezomib and dexamethasone before autologous stem cell transplantation according to IFM 2005-01 trial. Leukemia. 2010;24:1233–1235. doi: 10.1038/leu.2010.82. [DOI] [PubMed] [Google Scholar]
- 74.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zingone A, Kwok ML, Manasanch EE, et al. Phase II Clinical and Correlative Study Of Carfilzomib, Lenalidomide, and Dexamethasone Followed By Lenalidomide Extended Dosing (CRD-R) Induces High Rates Of MRD Negativity In Newly Diagnosed Multiple Myeloma (MM) Patients. Blood. 2013;122:538. [Google Scholar]
- 76.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. The New England journal of medicine. 2014;371:906–917. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 77.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 78.Mateos M-V, Richardson PG, Schlag R, et al. Bortezomib Plus Melphalan and Prednisone Compared With Melphalan and Prednisone in Previously Untreated Multiple Myeloma: Updated Follow-Up and Impact of Subsequent Therapy in the Phase III VISTA Trial. J Clin Oncol. 2010;28:2259–2266. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 79.Palumbo A, Hajek R, Delforge M, et al. Continuous Lenalidomide Treatment for Newly Diagnosed Multiple Myeloma. N Engl J Med. 2012;366:1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 80.Stewart AK, Jacobus S, Fonseca R, et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood. 2015;126:1294–1301. doi: 10.1182/blood-2014-12-613927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roy V, Stewart AK, Bergsagel PL, et al. Phase I/II study of melphalan, prednisone and lenalidomide combination for patients with newly diagnosed multiple myeloma who are not candidates for stem cell transplantation. Blood Cancer J. 2015;5:e294. doi: 10.1038/bcj.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palumbo A, Bringhen S, Rossi D, et al. Overall Survival Benefit for Bortezomib-Melphalan-Prednisone-Thalidomide Followed by Maintenance with Bortezomib-Thalidomide (VMPT-VT) Versus Bortezomib-Melphalan-Prednisone (VMP) in Newly Diagnosed Multiple Myeloma Patients. ASH Annual Meeting Abstracts. 2012;120:200. [Google Scholar]
- 83.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 84.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 85.Blade J, Vesole DH, Gertz M. Transplantation for multiple myeloma: who, when, how often? Blood. 2003;102:3469–3477. [Google Scholar]
- 86.Kumar A, Loughran T, Alsina M, Durie BG, Djulbegovic B. Management of multiple myeloma: a systematic review and critical appraisal of published studies. Lancet Oncology. 2003;4:293–304. doi: 10.1016/s1470-2045(03)01077-5. [DOI] [PubMed] [Google Scholar]
- 87.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 88.Facon T, Mary JY, Harousseau JL, et al. Front-line or rescue autologous bone marrow transplantation (ABMT) following a first course of high dose melphalan (HDM) in multiple myeloma (MM). Preliminary results of a prospective randomized trial (CIAM) protocol. Blood. 1996;88(Suppl 1):685a. [Google Scholar]
- 89.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. Journal of Clinical Oncology. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 90.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma.[see comment] N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 91.Cavo M, Tosi P, Zamagni E, et al. Prospective, Randomized Study of Single Compared With Double Autologous Stem-Cell Transplantation for Multiple Myeloma: Bologna 96 Clinical Study. J Clin Oncol. 2007;25:2434–2441. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 92.Fermand JP, Alberti C, Marolleau JP. Single versus tandem high dose therapy (HDT) supported with autologous blood stem cell (ABSC) transplantation using unselected or CD34-enriched ABSC: results of a two by two designed randomized trial in 230 young patients with multiple myeloma (MM) Hematol J. 2003;4(Suppl 1):S59. Hematol J 2003;4(Suppl 1):S59. [Google Scholar]
- 93.Goldschmidt H. Single vs. tandem autolgous transplantation in multiple myeloma: the GMMG experience. Hematol J. 2003;4(Suppl 1):S61. [Google Scholar]
- 94.Stewart AK. Reduced-intensity allogeneic transplantation for myeloma: reality bites. Blood. 2009;113:3135–3136. doi: 10.1182/blood-2008-12-173526. [DOI] [PubMed] [Google Scholar]
- 95.Attal M, Harousseau J-L, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma 10.1182/blood-2006-05-022962. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 96.Spencer A, Prince HM, Roberts AW, et al. Consolidation Therapy With Low-Dose Thalidomide and Prednisolone Prolongs the Survival of Multiple Myeloma Patients Undergoing a Single Autologous Stem-Cell Transplantation Procedure. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.18.8573. JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 97.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. N Engl J Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 98.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after Stem-Cell Transplantation for Multiple Myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajkumar SV. Haematological cancer: Lenalidomide maintenance-perils of a premature denouement. Nat Rev Clin Oncol. 2012 doi: 10.1038/nrclinonc.2012.100. [DOI] [PubMed] [Google Scholar]
- 100.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clinic Proceedings. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 101.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dimopoulos MA, Swern AS, Li JS, et al. Efficacy and safety of long-term treatment with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood Cancer J. 2014;4:e257. doi: 10.1038/bcj.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Richardson PG, Xie W, Jagannath S, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014;123:1461–1469. doi: 10.1182/blood-2013-07-517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826–1832. doi: 10.1182/blood-2013-11-538835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bringhen S, Petrucci MT, Larocca A, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. 2014 doi: 10.1182/blood-2014-03-563759. [DOI] [PubMed] [Google Scholar]
- 106.Jakubowiak AJ. Evolution of carfilzomib dose and schedule in patients with multiple myeloma: A historical overview. Cancer Treat Rev. 2014;40:781–790. doi: 10.1016/j.ctrv.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 107.Stadtmauer EA, Abonour R, Cohen AD, et al. Phase I/II Dose Expansion Of a Multi-Center Trial Of Carfilzomib and Pomalidomide With Dexamethasone (Car-Pom-d) In Patients With Relapsed/Refractory Multiple Myeloma. Blood. 2013;122:690. [Google Scholar]
- 108.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 109.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. The New England journal of medicine. 2015;372:142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 110.Dimopoulos Meletios A, Moreau Philippe, Palumbo Antonio, et al. Carfilzomib and dexamethasone (Kd) vs bortezomib and dexamethasone (Vd) in patients (pts) with relapsed multiple myeloma (RMM): Results from the phase III study ENDEAVOR. Journal of Clinical Oncology, 2015 ASCO Annual Meeting. 2015;33:8509. [Google Scholar]
- 111.San-Miguel MDJF, Hungria VTM, Yoon MDS. Randomized phase 3 trial of the deacetylase inhibitor panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in relapsed or relapsed and refractory multiple myeloma. Lancet Oncol. 2014;15:1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 112.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. The New England journal of medicine. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 113.Moreau Philippe, Masszi Tamás, Grzasko Norbert, et al. Ixazomib, an Investigational Oral Proteasome Inhibitor (PI), in Combination with Lenalidomide and Dexamethasone (IRd), Significantly Extends Progression-Free Survival (PFS) for Patients (Pts) with Relapsed and/or Refractory Multiple Myeloma (RRMM): The Phase 3 Tourmaline-MM1 Study ( NCT01564537). ASH Annual Meeting Abstracts.2015. [Google Scholar]
- 114.Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) Plus Low-Dose Dexamethasone As Therapy for Relapsed Multiple Myeloma. J Clin Oncol. 2009;27:5008–5014. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- 115.Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM) Leukemia. 2010;24:1934–1939. doi: 10.1038/leu.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lacy MQ, Allred JB, Gertz MA, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118:2970–2975. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vij R, Wang M, Kaufman JL, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119:5661–5670. doi: 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.San-Miguel JF, Richardson PG, Gunther A, et al. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3696–3703. doi: 10.1200/JCO.2012.46.7068. [DOI] [PubMed] [Google Scholar]
- 121.Sagar Lonial, Weiss Brendan M, Saad Zafar Usmani, et al. Phase II study of daratumumab (DARA) monotherapy in patients with ≥ 3 lines of prior therapy or double refractory multiple myeloma (MM): MMY2002 (Sirius) J Clin Oncol. 2015;33 (suppl; abstr LBA8512). [Google Scholar]


