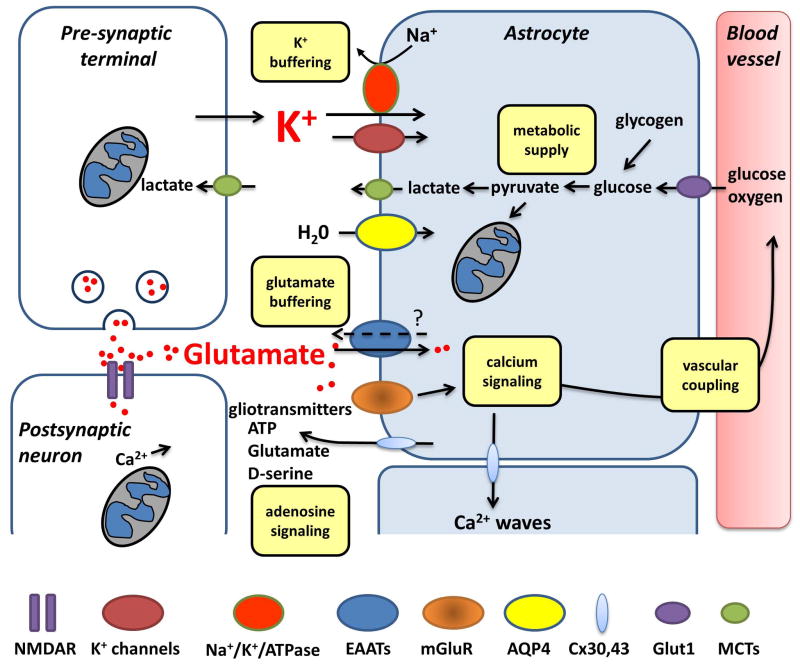
Figure 1. Major mechanisms by which astrocytes can participate in spreading depolarization (SD).
Waves of SD involve large extracellular K+ and glutamate increases, as well as severe demands on ATP production. Astrocyte uptake of K+ (via channels and the activity of Na+/K+/ATPase) can delay the onset or progression of SD, and participate in recovery of tissues after SD. Glutamate uptake by astrocytes could also limit the spread of SD, and prevent excessive accumulation of glutamate and excitotoxic NMDA receptor activation. It is not yet known to what degree reverse operation of excitatory amino acid transporters (EAATs) or vesicular release of glutamate from astrocytes may contribute SD or its consequences. Astrocyte metabolism is critical for transport activities, and it is also possible that lactate from astrocytes could provide an energy source for neurons during recovery from SD. Astrocytes could serve as a significant source of extracellular adenosine, which is enhanced in the extracellular space following SD. Adenosine could be derived as a consequence of intracellular depletion of ATP and transport of adenosine via equilibrative transporters, or ATP release and degradation by ecto-ATPase activity. Astrocyte Ca2+ waves are prominent during SD, and are expected to modulate the dramatic vascular dynamics that are associated with SD, and could also regulate availability of the co-agonist required for NMDAR activation (D-serine). Gap junctions composed of connexins (Cx30 and Cx43) can mediate release of ATP and glutamate, and also allow intercellular passage of mediators such as IP3. The importance of glial-derived ATP and glutamate for the propagation of astrocyte Ca2+ waves is not yet clear for SD. Astrocytes are well established to swell substantially following SD, involving water flux through aquaporin 4 channels (AQP4). However, it is important to keep in mind that the ionic fluxes and metabolic demands of SD are much greater than those observed during regulated synaptic transmission, implying that astrocyte roles described under other recording conditions may not directly apply to the unusual circumstances of SD.