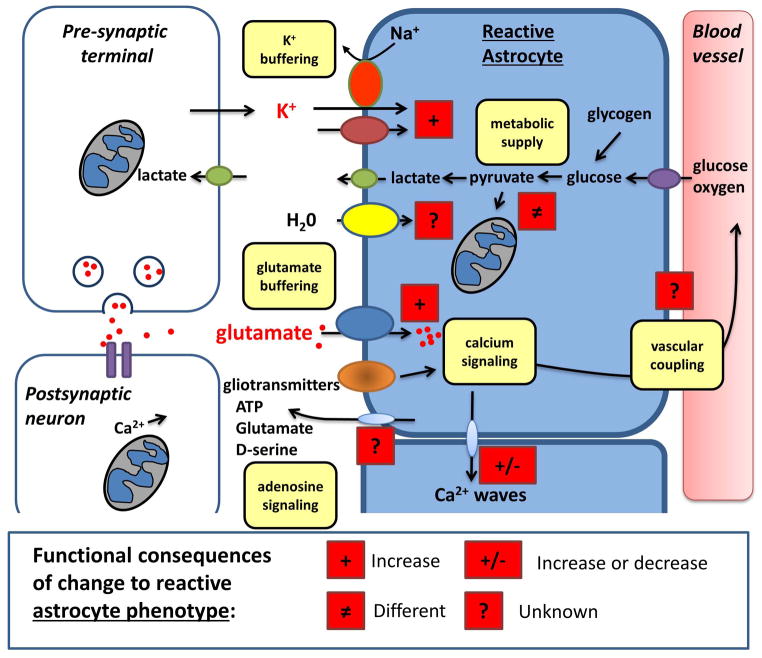
Figure 2. Modifications of function in reactive astrocytes.
Following conversion to a reactive phenotype, the functional properties of astrocytes can be significantly modified in ways that are relevant to SD and its deleterious consequences. The symbols used in this figure follow the key provided in Figure 1, and possible differences from functions in Figure 1 are highlighted with red boxes. Buffering of extracellular K+ may be increased sufficiently to raise the threshold for SD initiation or propagation. Likewise, increased accumulation of glutamate from the extracellular space may limit the spread of SDs, as well as reducing the likelihood of NMDAR-dependent toxicity. Reactive astrocytes undergo modifications in energy metabolism, which could render them more resistant to the metabolic challenges of SD in the context of various brain injuries. There is currently a notable lack of information on how changing to a reactive phenotype modifies other key astrocyte functions related to SD. For example, it is not known whether astrocyte Ca2+ waves are modified, and whether this could be significant for the critical issue of disrupted neurovascular coupling that accompanies SD in injured brain tissue. There is also a lack of information on the influence on cell swelling mechanisms, ionic homeostasis, and possible release of gliotransmitters. Some of these modifications seen in reactive astrocytes could be targeted to reduce the number of SDs and/or reduce their deleterious consequences in injured brain.