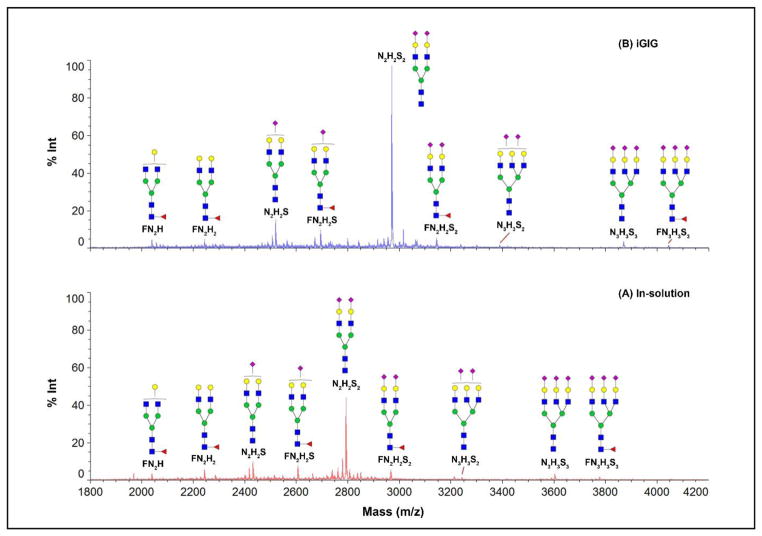
Figure 8. MALDI-MS spectra of permethylated N-glycans from human serum.
N-glycans are prepared in solution (A) and on solid-phase (B) using 20 μL serum. In the in-solution method, serum is directly digested using PNGase F after protein denaturation; the released N-glycans are then permethylated. On solid-phase, glycoproteins are immobilized on beads for glycan modification, e.g., sialic acid stabilization before permethylation. The top nine N-glycans are listed, in which iGIG yields more N-glycans based on their respective intensities. The same m/z is observed on glycans that do not contain sialic acids. A higher intensity is observed on sialylated glycans due to their protection.