Figure 4.
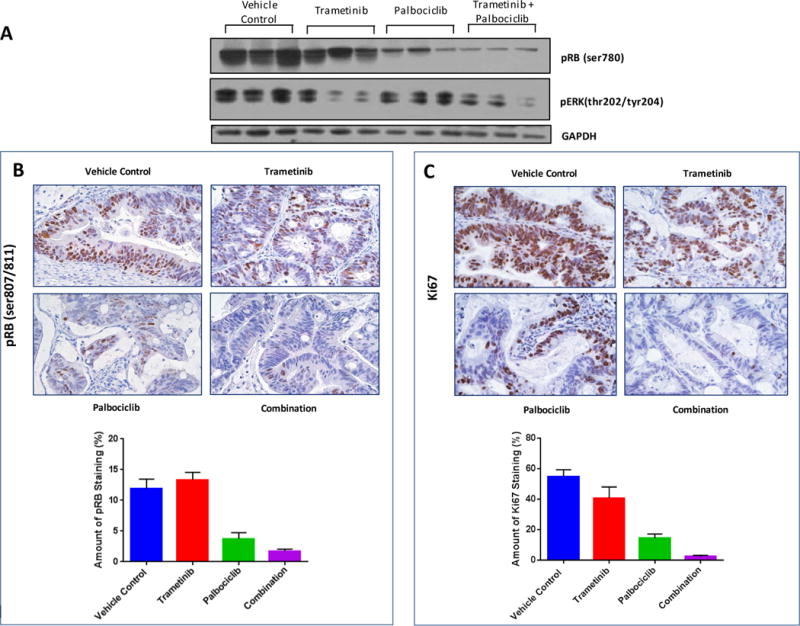
(A) Immunoblot analysis of pRB (ser780) and pERK (thr202/tyr204) in CRM 13-180 tumors harvested from the efficacy experiment. Tumors were harvested at two hours following the last treatment, lysed and probed with the indicated antibodies. (B) Immunohistochemical staining of pRB in CRM 13-180 tumors. A subsequent study (no efficacy component) was carried out to generate additional tumors for IHC analysis. Mice bearing subcutaneous CRM 13-180 tumors were treated by oral gavage for 10 days with vehicle, trametinib at 3mg/kg, palbociclib at 150mg/kg, or the combination at the single agent doses. Tumors were harvested at two hours following the last treatment. Tumors were stained for pRB (ser807/811) expression (top) and the amount of staining was quantitated. (C) Tumors were also stained for Ki67 (top) and the amount of staining was quantitated (bottom). P = 0.0191 (vehicle versus trametinib) and p ≤ 0.0001 for all other group comparisons.
