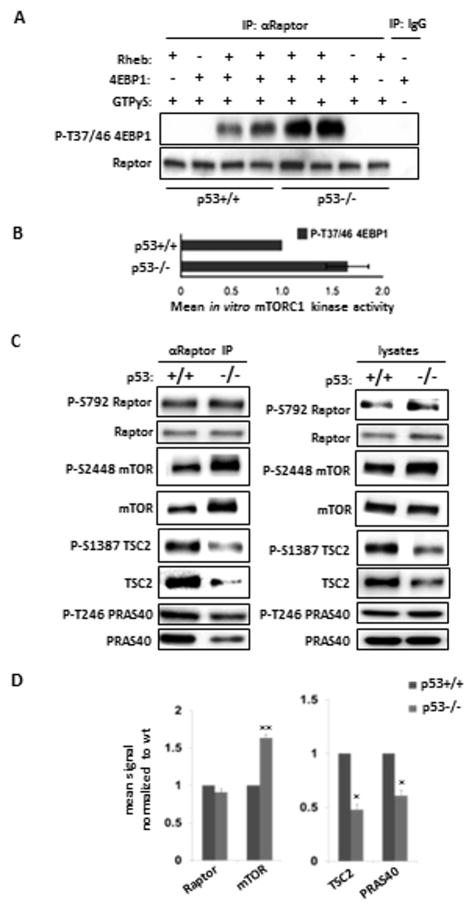
Figure 3. The enhanced kinase activity of the mTORC1 complex is retained during immunoprecipitation in cells lacking p53.
(A) The mTORC1 complex from p53−/− cells has enhanced kinase activity in vitro. mTORC1 was immunoprecipitated with an anti-Raptor antibody under low salt (100 mM) conditions and in vitro kinase assays were performed using 4EBP1 as a substrate; p-T37/46 4EBP1 generated was determined by immunoblot. (B) Licor densitometry data from 3 independent in vitro kinase assays (mean ±sd). (C) The components of the mTORC1 complex differ between p53+/+ and p53−/− cells. Anti-Raptor immunoprecipitates (IPs) from isogenic HCT116 cells were probed with antibodies against the indicated proteins; lysates were probed in parallel. Equivalent IPs using IgG that were processed and exposed equally were uniformly blank (not shown). (D) The level of Raptor, mTOR, PRAS40, and TSC2 in p53-null HCT116 cells was measured by densitometry from 3 independent experiments and expressed (±sd) relative to the level in p53 wt control cultures.**p=0.0045 for mTOR, *p=0.048 for TSC2, *p=0.0117 for PRAS40.