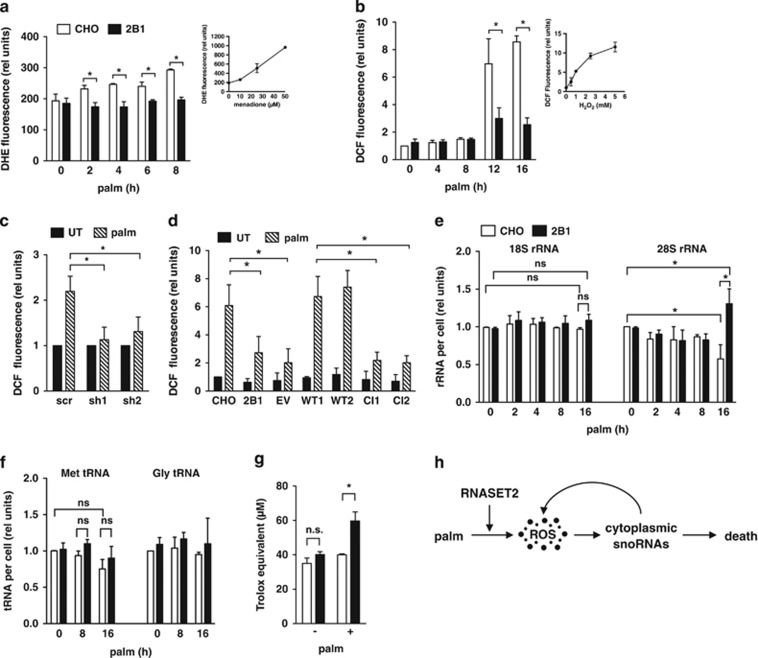
Figure 5.
RNASET2 is required for induction of ROS during lipotoxicity. (a, b) CHO (open bars) and 2B1 (filled bars) cells were treated with palmitate for the indicated times. ROS generation was quantified by diyhdroethidium (DHE) staining and fluorescence microscopy (a) or by 2′,7′-dichlorodihydrofluorescein diacetate (DCF) staining with flow cytometry analysis of 104 cells/sample (b). Insets show controls for detection of superoxide (a, DHE staining of CHO cells treated with menadione) and H2O2 (b, DCF staining of CHO cells treated with H2O2). (c) C2C12 clonal lines transfected with shRNA sequences targeting RNASET2 or with scrambled (scr) control shRNA were treated with palm for 16 h. ROS was quantified by DCF staining and flow cytometry analysis in untreated (filled bars) and palm-treated (hatched bars) cells. (d) Mutant 2B1 clonal lines transfected with EV or plasmids encoding WT or CI murine RNASET2 were treated with 500 μM palm for 12 h. ROS was quantified by DCF staining and flow cytometry. (e, f) Total RNA was isolated from palm-treated CHO (open bars) and 2B1 (filled bars) cells. Reverse transcription with random hexamers (e) or with target-specific stem-loop primers (f) and qPCR was used to quantify rRNAs and tRNAs, respectively. (g) CHO (open bars) and 2B1 (filled bars) cells were untreated or treated with 500 μM palm for 8 h. Antioxidant capacity of cell lysates (50 μg protein) was quantified by Trolox equivalent antioxidant capacity (TEAC) assay. (h) Following exposure to lipotoxic conditions (palm), ROS is generated in an RNASET2-dependent manner. Downstream of ROS, rpL13a snoRNAs accumulate in the cytosol, and promote amplification of ROS. These events contribute to cell death. All graphs report mean+S.E. for n=3 independent experiments. *P<0.05 for comparisons indicated; ns, non-significant