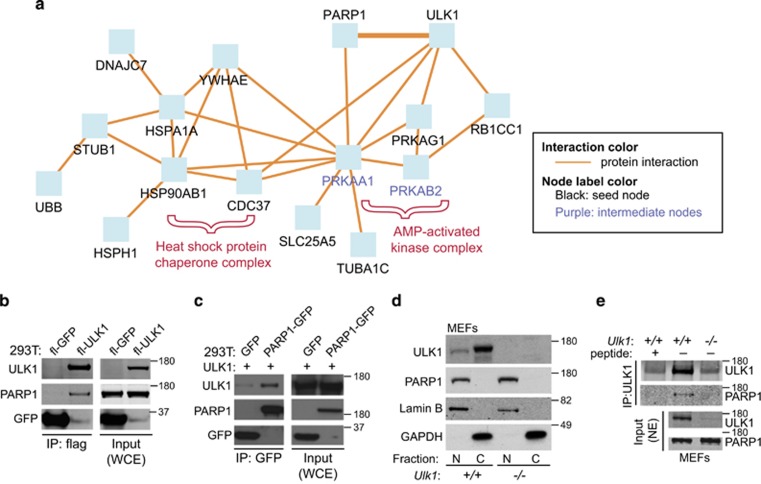
Figure 3.
ULK1 interacts with PARP1. (a) PARP1 is identified as a putative ULK1-interacting partner by using an unbiased proteomics-based approach. Proteins significantly (P<0.05) enriched in the flag-ULK1 immunoprecipitates compared with control immunoprecipitates (Table 1) were submitted to ConsensusPathDB for induced network-module gene-set analysis to visualize previously described relationships among the proteins. Interactions between ULK1 and the HSP90 chaperone complex, AMPK subunits, 14-3-3, and RB1CC1 have been previously validated.23, 27(b) Whole-cell extracts (WCEs) and anti-flag immunoprecipitates from 293T cells transfected with Flag-GFP or Flag-ULK1 were subjected to immunoblot analyses by using antibodies against the indicated proteins (PARP1, ULK1, and GFP). PARP1 was detected in the Flag-ULK1 immunoprecipitates but not in Flag-GFP immunoprecipitates. (c) WCEs and anti-GFP immunoprecipitates from 293 T cells cotransfected with Flag-ULK1 and either GFP or PARP1-GFP were subjected to immunoblot analyses by using antibodies against the indicated proteins. ULK1 was more abundant in the PARP1-GFP immunoprecipitates than in the GFP immunoprecipitates. (d) Nuclear (N) and cytosolic (C) fractions were prepared from Ulk1+/+ and Ulk1−/− MEFs and subjected to immunoblot analyses using antibodies against PARP1, GAPDH, and ULK1. ULK1 immunoreactivity in the nuclear-enriched fraction shows the presence of ULK1 in the nucleus. (e) Nuclear fractions prepared from Ulk1+/+ and Ulk1−/− MEFs were subjected to IP with an anti-ULK1 antibody (N17, Santa Cruz) in the presence or the absence of an antibody-specific blocking peptide. Endogenous PARP1 immunoprecipitated with endogenous ULK1 from nuclear extracts prepared from WT MEFs