Abstract
Toxic epidermal necrolysis (TEN) is a disease which is characterized by fever and desquamation of the skin and mucosal membranes. It is usually related with drugs, especially aromatic anticonvulsants which are recognized as the most common cause of this disorder. Cranial irradiation may act as a precipitating factor along with anticonvulsants for the development of TEN. We report a 28-year-old patient with central nervous system (CNS) relapsed non-Hodgkin lymphoma (NHL) who developed TEN after cranial radiotherapy and concurrent phenytoin treatment.
Keywords: Radiotherapy, Fenitoin, Toxic epidermal necrolysis
1. Introduction
Toxic epidermal necrolysis (TEN) is a life-threatening disease which is characterized by fever and extensive epidermal desquamation complicated by multiorgan dysfunction.1, 2 Patients could loose almost 90% of their body skin surface and fatal complications, like severe electrolyte imbalance, renal impairment and sepsis, could be seen. Most important etiological agents are drugs. Although any drug could trigger TEN, some patients on cranial radiotherapy and phenytoin treatment could develop severe cutaneous hypersensitive drug reaction that may manifest as TEN.3, 4 Occasionally, the underlying reason could not be detected due to multiple medications. Although most cases of TEN occur in males, our patient is female.5
2. Case
Twenty-eight-year old woman was diagnosed with primary mediastinal diffuse large B cell lymphoma (DLBCL) by biopsy of mediastinal bulky mass in April 2012. Six courses of standard doses R-CHOP regimen (Rituximab, cyclophosphamide, doxorubicin, vincristin, prednisone) were administered and completed in September 2012 with complete remission (CR). After 2 months from the last dose of chemotherapy, she was presented to emergency service with symptoms and signs of epilepsy. Levetiracetam treatment was started to control seizures. Cranial magnetic resonance imaging showed multiple lesions in around the left and right lateral ventricles and temporal horn. Stereotaxic biopsy was performed from a mass in the left anterior frontal lob and DLBCL was identified. She was treated with standard dose of IDARAM (idarubicin, dexamethasone, methotrexate) regimen. But, after first cycle of chemotherapy, tonic–clonic epileptic seizures were detected and phenytoin was added to levetiracetam to control the seizures. Although the seizures were controlled with phenytoin, bullous skin lesions first appeared at the scalp at the 3rd day of whole cranial radiotherapy and 12th day of phenytoin therapy and generalized to whole body (Fig. 1, Fig. 2). She had edematous lips with necrotic mucous membranes and oral ulcerations. Dermatological examination revealed the diagnosis of TEN and skin shave biopsy from the right femoral was performed. Lymphocytic infiltration of the dermo-epidermal junction, cell necrosis in epidermis and bullous formation in subepidermis was identified from the biopsy. All medications were stopped and wet-patches were applied to the whole skin surface and local skin care with antibiotic and antifungal dressings. Due to malnutrition and electrolyte imbalance, parenteral nutrition was given and electrolyte balance was obtained by appropriate treatment. She was managed with systemic antibiotics and methylprednisolone (the dose of 1 mg/kg). After supportive care her condition worsened, she manifested septicemia and hemodynamic instability and died after 3 weeks on phenytoin.
Fig. 1.
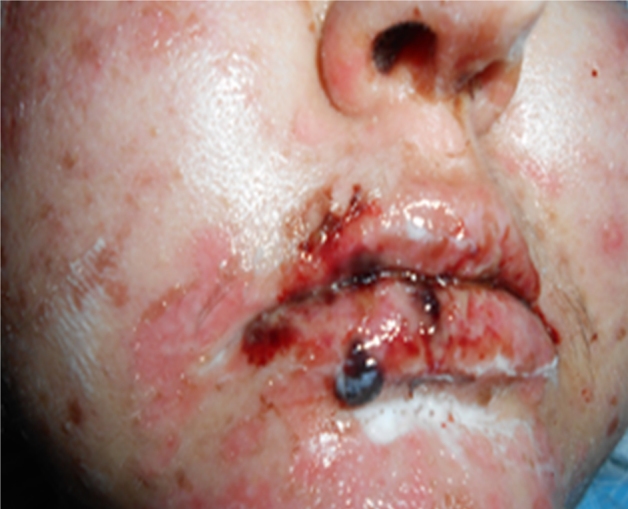
Necrolysis on oral mucosal membrane.
Fig. 2.
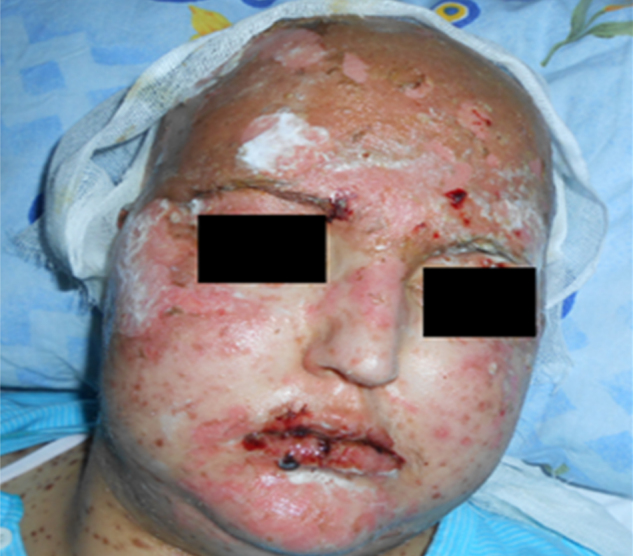
Epidermal desquamation of skin.
3. Conclusion
Although it is very rare, TEN is one of the most severe and serious complication of drugs, with anticonvulsants such as phenytoin, carbamazepine, and phenobarbital, most commonly implicated. In addition, allopurinol, NSAIDs (paracetamol and nimesulide are most common), antibiotics, have also been found to have a role.6 TEN typically begins 1–3 weeks after the initiation of therapy but occurs more rapidly with drug rechallenge or in the presence of provoking factors. TEN is a special form of hypersensitivity reaction and treatment should be based on treatment of the underlying cause. Radiation therapy may cause this disease.7 However, concurrent use of whole brain RT and phenytoin has been shown to induce cutaneous drug reactions such as TEN.3, 8 The first case was reported by Delattre et al. in 1988 reporting a patient on radiation therapy and phenytoin who developed drug reaction. This syndrome is a life-threatening condition. Severity-of-illness scale (SCORTEN) is a useful method to predict mortality in TEN patients (Table 1).9 Our presented case had multiple risk factors, which made her prognosis very poor. According to the SCORTEN scale, she had a total of 4 positive factors (TBSA involved >10%, HR >100, serum BUN >28 and a malignancy), corresponding to 58.3% mortality rate. Clinics should be aware of this and patients who have to receive radiation therapy should avoid unnecessary anticonvulsants. The appropriate and quick action should be taken as soon as TEN is detected, especially in cancer patients due to its high morbidity and mortality rates.
Table 1.
Severity-of-illness score for toxic epidermal necrolysis (SCORTEN) [9].
| Risk factor* | Score 0 | Score 1 | Our patients score |
|---|---|---|---|
| Age | <40 yr | ≥40 yr | 0 |
| Associated cancer | No | Yes | 1 |
| Heart rate (beats/min) (HR) | <120 | ≥120 | 1 |
| Serum BUN (mg/dL) | ≤ 28 | >28 | 1 |
| Detached or compromised body surface | <10% | ≥10% | 1 |
| Serum bicarbonate (mEq/L) | ≥20 | <20 | 0 |
| Serum glucose (mg/dL) | ≤250 | >250 | 0 |
| *More risk factors indicate a higher score and a higher mortality rate (%) as follows: | Total | ||
| 0–1 = 3.2% | 4 | ||
| 2 = 12.1% | |||
| 3 = 35.3% | |||
| 4 = 58.3% | |||
| ≥5 = >90% | |||
Conflict of interest
None declared.
Financial disclosure
None declared.
Contributor Information
Fatma Keklik, Email: fatma_keklik86@hotmail.com.
Melda Cömert Özkan, Email: meldacomert@hotmail.com.
Gizem Kocabaş Yenipazar, Email: gizem.kocabas@ege.edu.tr.
Banu Yaman, Email: banu.yaman@ege.edu.tr.
Güray Saydam, Email: guray.saydam@ege.edu.tr.
Fahri Şahin, Email: fahri.sahin@ege.edu.tr.
References
- 1.Harr T., French L.E. Toxic epidermal necrolysis and Stevens–Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi: 10.1186/1750-1172-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz R.A., McDonough P.H., Lee B.W. Toxic epidermal necrolysis. Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173e1–173e13. doi: 10.1016/j.jaad.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Mockenhaupt M., Messenheimer J., Tennis P., Schlingmann J. Risk of Stevens–Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology. 2005;64:1134–1138. doi: 10.1212/01.WNL.0000156354.20227.F0. [DOI] [PubMed] [Google Scholar]
- 4.Delattre J.Y., Safai B., Posner J.B. Erythema multiforme and Stevens–Johnson syndrome in patients receiving cranial irradiation and phenytoin. Neurology. 1988;38:194–198. doi: 10.1212/wnl.38.2.194. [DOI] [PubMed] [Google Scholar]
- 5.Aguiar D., Pazo R., Duran I. Toxic epidermal necrolysis in patients receiving anticonvulsants and cranial irradiation: a risk to consider. J Neurooncol. 2004;66:345–350. doi: 10.1023/b:neon.0000014538.31561.bc. [DOI] [PubMed] [Google Scholar]
- 6.Patel T.K., Barvaliya M.J., Sharma D., Tripathi C. A systematic review of the drug-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J Dermatol Venereol Leprol. 2013;79(3):389–398. doi: 10.4103/0378-6323.110749. [DOI] [PubMed] [Google Scholar]
- 7.Roujeau J.C., Kelly J.P., Naldi L., Rzany B., Stern R.S., Anderson T. Medication use and the risk of Stevens–Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–1607. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 8.Aydoğan K., Vatansever S., Adim S.B., Saricaoglu H. Empact syndrome: a case report and review of the literature. Int J Dermatol. 2010;49:945–949. doi: 10.1111/j.1365-4632.2009.04338.x. [DOI] [PubMed] [Google Scholar]
- 9.Bastuji-Garin S., Fouchard N., Bertocchi M., Roujeau J.C., Revuz J., Wolkenstein P. SCORTEN: a severity-of illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–153. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]


