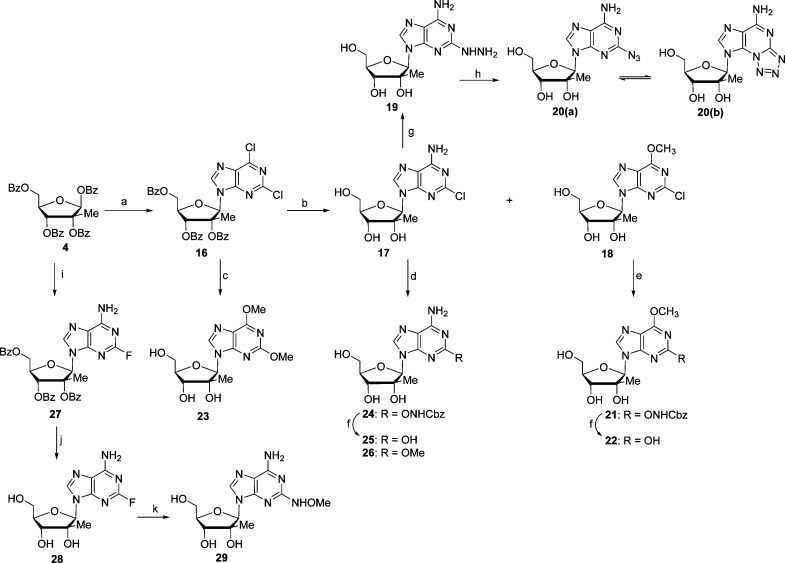
Scheme 3.
Reagents and conditions: (a) 2,6-dichloropurine, DBU, TMSOTf, −40 to 80 °C, 4 h, 80%; (b) sat. NH3/MeOH, rt, 3 days; for 17, 46%; for 18, 21%; (c) K2CO3, MeOH, rt, 24 h, 87%; (d) for 24, HONHCbz, NaH, THF, 50 °C, 24 h, 81%; for 26, MeONa, MeOH, 65 °C, 24 h, 92%; (e) HONHCbz, NaH, THF, 50 °C, 24 h, 78%; (f) Pd/C, H2, MeOH, rt, 15 h; for 22, 83%; for 25, 97%; (g) NH2NH2, MeOCH2CH2OH, 110 °C, 5 h, 40%; (h) NaNO2, HOAc, 1 h, 77%; (i) 2-fluoroadenine, DBU, TMSOTf, −40 to 65 °C, 5 h; (j) sat. NH3/MeOH, rt, 2 d, 72% for two steps; (k) MeONH2, Et3N, EtOH/H2O, 110 °C, 15 h, 66%.