Scheme 2.
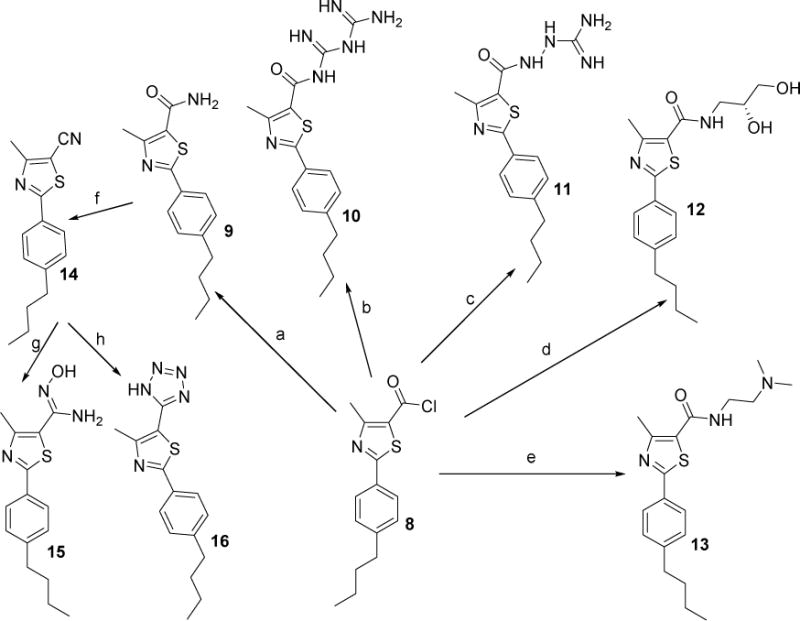
Reagents and conditions: (a) 30 % aq NH4OH, THF, rt, 24 h; (b) biguanidine hydrochloride, Et3N, THF, 24 h; (c) aminoguanidine hydrochloride, Et3N, THF, 24 h; (d) (R)-(−)-3-amino-1,2-propanediol,THF, rt, 24 h; (e) N,N-dimethylethylenediamine, THF, rt, 48 h; (f) thionyl chloride, reflux, 7 h; (g) NH2OH HCI, K2C03, EtOH, 78 °C, 24 h; (h)NaN3, l2, DMF, 120°C, 15h.
