Figure 3. Targeting translation in melanoma.
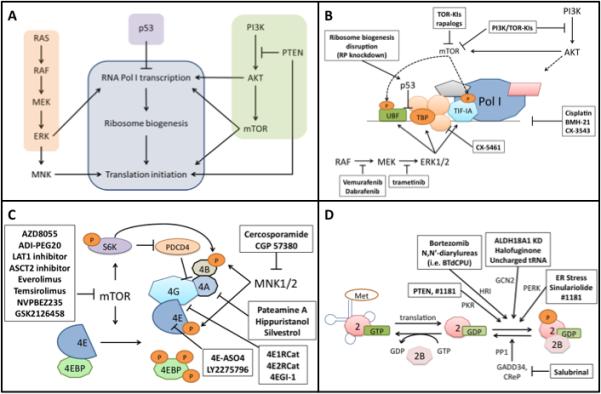
(A) Diagram of how the MAPK pathway, PI3K pathway, and p53 modulate protein synthetic machinery. (B) Pol I transcription can be regulated by many different mechanisms. This figure depicts the regulatory methods most relevant to melanoma. UBF, TBP, and TIF-IA can all be regulated by the MAPK pathway via ERK1/2. The PI3K/AKT/mTOR pathway regulates transcription through the phosphorylation of UBF and TIF-IA. AKT also regulates Pol I transcription through mTORC1-independent mechanisms. p53 is able to impair Pol I transcription by inhibiting SL1 binding to UBF. Nonspecific inhibitors of Pol I transcription include cisplatin, BMH-21, and CX-3543 which disrupt transcription through activity on genomic DNA. Drugs targeting signaling pathways can also exert effect through disruption of Pol I transcription. BRAF and MEK inhibitors impair ERK1/2 ability to activate UBF, TBP, and TIF-IA. TOR kinase inhibitors (TOR-KIs) and rapalogs impair the ability of mTOR to activate UBF and TIF-IA. PI3K/TOR-KIs also prevent feedback activation of PI3K by mTOR inhibition and prevent mTORC1-independent Pol I activation by AKT. CX-5461 acts independently of mTOR and MAPK pathways by preventing SL1 complex association. (C) eIF4F complex inhibition. mTOR regulates eIF4F activity through 4EBP1 and p70S6K. Rapalogs, TOR-KIs, PI3K/TOR-KIs, and amino acid starvation can all inhibit mTOR activity, thus reducing mRNA translation. Regulation of translation through the MAPK pathway can be inhibited at the point of MNK1/2 by cercosporamide. Multiple drugs targeting the eIF4F complex are currently under investigation as potential cancer therapeutics. These include eIF4A inhibitors, inhibitors of eIF4E/eIF4G interaction, eIF4E cap-binding activity, and antisense oligonucleotide inhibitors of eIF4E. Furthermore, the biogenesis of 60S and 40S subunits necessary for mRNA translation can be impaired via ribosomal protein (RP) knockdown, or various Pol I disruptors. (D) Ternary complex inhibition. Activation of any of the eIF2α kinases causes phosphorylation of eIF2α, leading to sequestration of eIF2B, preventing GEF activity needed to continue translation. Many stresses can lead to kinase activation. Amino acid starvation or the mimicking of uncharged tRNA can activate GCN2. ER stress is a common activator of PERK. N,N’-diarylureas are activators of HRI, and PTEN activity independent of PI3K/AKT can lead to PKR activation. In addition, inhibitors of eIF2α phosphatase activity such as salubrinal can block eIF2B function.
