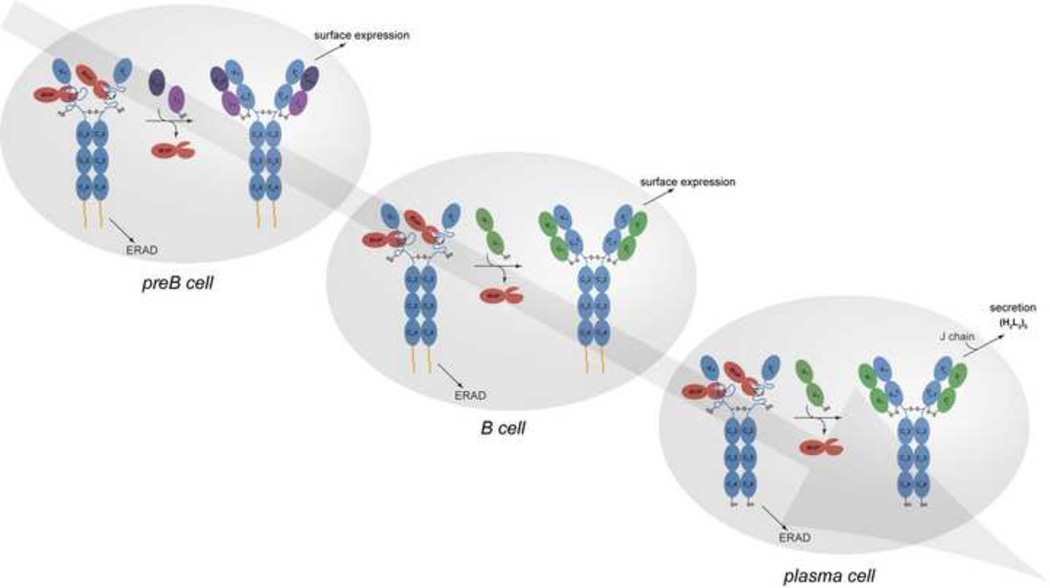
Figure 3. Immunoglobulin quality control checkpoints at various stages in B cell development.
After HC gene rearrangements, preB cells produce IgM HCs (μ HCs) (blue) bound to BiP (red). If their association with the surrogate LC, which is assembled from the VpreB (deep purple) and λ5 (light purple) proteins, induces BiP release and folding of the CH1 domain, and if the other Ig domains fold properly, the HC can traffic to the plasma membrane and engage signalling molecules (HC membrane anchor shown in yellow). If there is a failure in any of these steps, the μ HCs become substrates for ER associated degradation (ERAD) and are retrotranslocated to the cytosol for degradation by the 26S proteasome. Once conventional LCs (green) are produced in the B cell, they assemble with μ HCs, displace BiP from the CH1 domain, and induce its folding. As the ability of all domains of the HC to fold properly upon assembly was tested at the preB cell stage, quality control at this stage monitors the pairing and folding of the V domains. Plasma cell differentiation leads to the synthesis of extremely high levels of antibodies. Because the ability of the specific HC and LC combination to assemble and fold properly was verified at the B cell stage of development, quality control at this point involves monitoring the completeness of Ig assembly, focusing on the LC-induced release of BiP from the CH1 domain and its concomitant folding. There is a shift to production of the secretory form of μ HC in plasma cells, which possess a terminal cysteine that is involved in assembly with J chain and pentamer formation. Thiol-mediated retention mechanisms monitor the redox state of this cysteine and prevent IgM monomers from being secreted86.