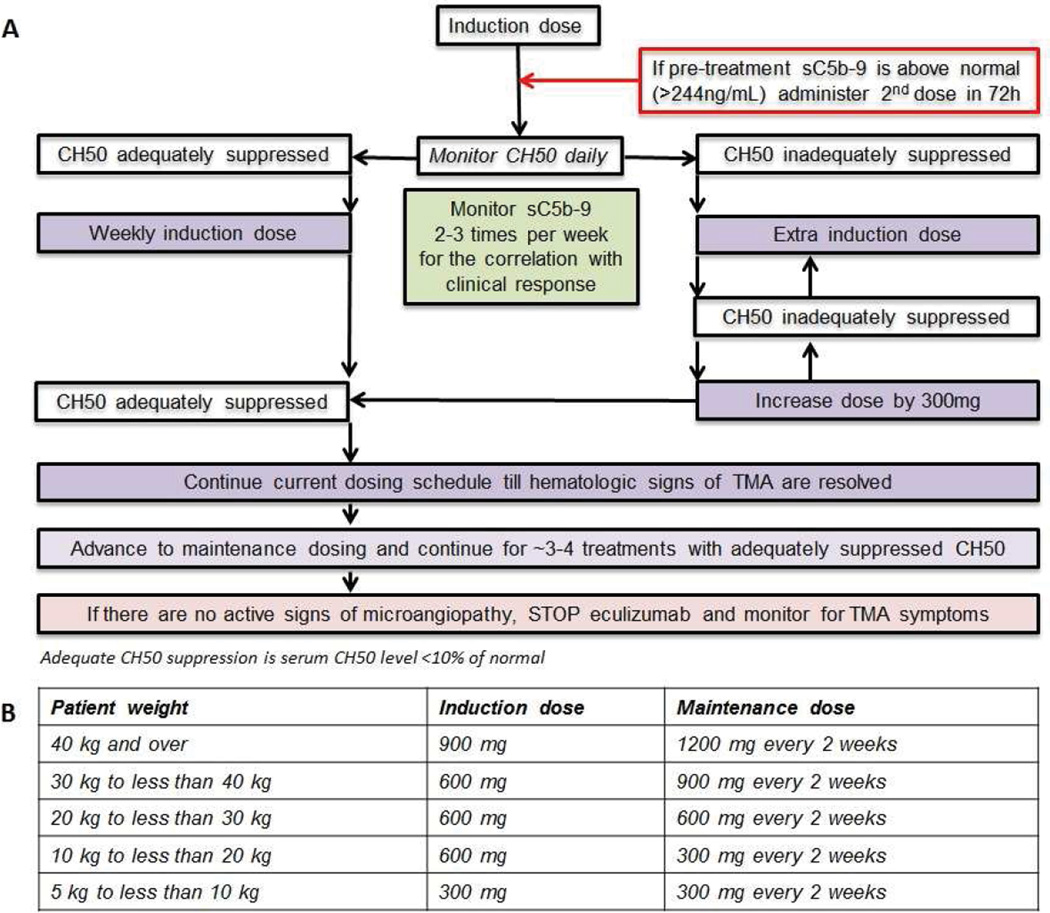
Figure 5. Eculizumab administration and monitoring schema for HSCT patients with TMA.
The first eculizumab dose for HSCT-associated TMA should be given based on patient’s weight, as listed in the table (B) according to the eculizumab package insert. If pre-treatment plasma sC5b-9 concentration is above normal >244 ng/mL, the second dose should be administered at no later than 72 hours. CH50 should be monitored each day during eculizumab induction therapy to determine the subsequent dosing schedule, since patients with TMA often require eculizumab dosing more often than weekly in the beginning of the induction therapy to sustain a therapeutic eculizumab serum concentration. To achieve complement blockade in the blood and to maintain therapeutic eculizumab serum concentration of ≥99 µg/mL, CH50 needs to remain adequately suppressed (less than 10% of the lower limit of normal). Subsequent eculizumab doses need to be given when CH50 becomes inadequately suppressed (rises above 10% of normal laboratory value), but no longer than every 7 day intervals. If CH50 remains inadequately suppressed by dosing at less than 7 day intervals and sC5b-9 remains elevated, dose should be increased by 300 mg/dose to the maximum of 1200 mg/dose and daily CH50 monitoring should continue. If CH50 is adequately suppressed for at least 6 days, then eculizumab induction doses should be given weekly. When steady CH50 suppression is achieved and hematologic TMA parameters and plasma sC5b-9 normalize, eculizumab should be advanced to a maintenance schedule as listed in table (B) based on patient’s weight, as recommended in eculizumab package insert. CH50 should be checked at least prior to each eculizumab dose to assure adequate complement blokade. If TMA remains controlled after 3–4 maintenance doses, eculizumab may be discontinued. Patients should be carefully monitored with twice a week LDH, CBC and differential, weekly urinalysis, and twice a week sC5b-9 for 4 weeks after eculizumab therapy is discontinued. Weekly CH50 should be checked until it returns to normal. Anti-meningococcal prophylaxis should be provided from the start of the therapy until about 8 weeks after stopping eculizumab and CH50 has returned to normal.