Abstract
The presence of monosomal karyotype (MK+) in acute myeloid leukemia (AML) is associated with dismal outcomes. We evaluated the impact of MK+ in AML (MK+AML, N=240) and in myelodysplastic syndrome (MK+MDS, N=221) on hematopoietic cell transplantation (HCT) outcomes compared to other cytogenetically defined groups (AML, N=3,360; MDS, N=1,373) as reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) from 1998 to 2011. MK+AML was associated with higher disease relapse (hazard ratio [HR] 1.98, p<0.01), similar transplant related mortality (TRM, HR 1.01, p=0.9) and worse survival (HR 1.67, p<0.01) compared to other cytogenetically defined AML. Among patients with MDS, MK+MDS was associated with higher disease relapse (HR 2.39, p<0.01), higher TRM (HR 1.80, p<0.01) and worse survival (HR 2.02, p<0.01). Subset analyses comparing chromosome 7 abnormalities (del7/7q) with or without MK+ demonstrated higher mortality for MK+ disease in for both AML (HR 1.72, p<0.01) and MDS (HR1.79, p<0.01). The strong negative impact of MK+ in myeloid malignancies was observed in all age groups and using either myeloablative or reduced intensity conditioning regimens. Alternative approaches to mitigate disease relapse in this population are needed.
Introduction
The presence of multiple chromosomal abnormalities, termed complex cytogenetics, in leukemia cells, is associated with unfavorable outcome. The reported definitions of complex cytogenetics varies from ≥3 to 5 cytogenetic abnormalities in a single clone(1, 2). Breems et al further examined this group of patients with poor risk disease and identified autosomal monosomies to be associated with poor outcome(3). This classification has a tighter association with poor outcome comparing to other non-random cytogenetic changes in the poor risk category and predicts a subset of patients with dismal outcome. The monosomal karyotype (MK) is defined as the presence of at least two autosomal monosomies or one autosomal monosomy associated with any other structure abnormality (MK+). Cytogenetic abnormalities have similar prognostic impact in myelodysplastic syndrome (MDS) where the number of chromosomal abnormalities is also associated with poor outcomes (4, 5) and in MDS, MK+ is strongly associated with shorter survival, similar to acute myeloid leukemia (AML) (6). In both AML and MDS, abnormalities in chromosome 7 including deletion and monosomy, are common single abnoermality associated with poor prognosis. The prognostic effect of MK+ could be due to single most common monosomy.
Hematopoietic cell transplantation (HCT) is the treatment of choice for patients with cytogenetic-defined poor risk AML in first complete remission (CR1), which may lead to 30 to 40% 5 year survival compared to <10% with non-transplant approaches(1, 7, 8). However, these data are mostly from patients younger than 60 years receiving allogeneic transplantation with myeloablative (MA) conditioning. Reduced intensity conditioning (RIC) is commonly used in AML patients older than 60 years (9). This reduction in intensity decreases toxicity and early transplant mortality allowing older or compromised patients to receive an allogeneic HCT. However, when comparing with MA approaches, this benefit is offset by increase in relapse rates(10). Additionally, a retrospective analysis done by the European Group for Blood and Marrow Transplantation (EBMT) demonstrated that poor risk cytogenetics at diagnosis is associated with higher relapse and shorter leukemia-free survival (LFS) in patients with AML in CR1 receiving RIC compared to myeloablative conditioning (11).
Older AML patients more often have increased cytogenetic abnormalities including unfavorable risk and MK(3, 12, 13). MK+ AML may increase the risk of relapse after transplantation(14–18) however it is unclear whether MA conditioning may mitigate this increased relapse risk. We analyzed the effect of MK+ AML in patients undergoing HCT in CR1 and explored the prognostic impact of the MK+ in transplants for MDS.
Materials and Methods
Data Sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) includes a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantation to a statistical center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; patients are followed longitudinally and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule(9).
Patients
All patients with AML in CR1 who received a first allogeneic HCT from 1998 to 2011 from HLA-matched or single HLA locus mismatched donors (8/8 or 7/8) were eligible for this study.
Patients with acute promyelocytic leukemia or evidence of t(15;17) as a sole cytogenetic abnormality, core binding factor AML, who received umbilical cord blood grafts, ex-vivo T-cell depleted grafts or patients with unknown cytogenetic information were excluded.
MK+AML was defined as the presence of two monosomies or one monosomy plus at least one other chromosome structural abnormality according to Breems et al(3). Cytogenetic abnormalities present at diagnosis and prior to initiation of conditioning regimen are reported to the CIBMTR. When required, additional review of reported cytogenetic data was performed by three reviewers (MCP, BCM and MB) to adjudicate any uncertainties in classification. Cases with incomplete data were classified as unknown cytogenetics and excluded.
Eligible AML patients were categorized into MK+ AML (N=240), AML other unfavorable (N=1138) and intermediate risk groups (2). The intermediate risk was further separated into normal karyotype (N=643) and intermediate risk with abnormal karyotype (AML-IRabn, N=1579). Eligible MDS patients were categorized into MK+MDS (N=221), MDS other unfavorable (N=423), normal karyotype (N=241), MDS- IRabn (N=611) and favorable karyotype (N=98)(4). MDS cases were also classified as early and advanced according to the CIBMTR definition(9). Subset analysis to compare abnormalities of chromosome 7 (monosomy or deletion) with or without meeting the MK+ definition was performed separately in AML and MDS divided as: MK+ with chromosome 7 abnormalities (MK/7abn, AML N=148, MDS N=171), chromosome 7 without MK+ (7abn, AML n=275, MDS n=304) and normal karyotype (AML N=643, MDS=241).
Study Endpoints and Variables
The cytogenetic groups were compared for the clinical endpoints of overall survival, disease-free survival (DFS), relapse and transplant related mortality (TRM). Overall survival included time from HCT until death from any cause and patients were censored at last follow up. DFS included death, leukemia or MDS relapse as a composite endpoint and patients were censored at last follow up. Relapse included any reported events of leukemia relapse. TRM was defined as death in the absence of prior leukemia [or MDS] relapse.
Variables analyzed in the multivariate model include: cytogenetic groups, age, performance score, conditioning regimen intensity(19), donor type, donor/recipient CMV serologic status, graft source, year of transplant, graft versus host disease (GVHD) prophylaxis, use of in vivo T-cell depletion (anti-thymocyte globulin [ATG] or alemtuzumab), planned use of any myeloid growth factor to promote engraftment (defined as any growth factor initiated within 12 days after the graft infusion). Conditioning intensity use was confounded by the age of the patient with RIC mostly utilized in patients older than 40 years. For the analysis age and conditioning intensity were combined into composite covariate groups as: 1) myeloablative (MA) < 21years, MA 21–40 years, MA 41–60 years, RIC 41–60 years, RIC 61–64 years, RIC ≥65 years.
Statistical Analysis
Probabilities of overall survival and DFS were calculated using the Kaplan-Meier estimator. Values for relapse and TRM were generated using cumulative incidence estimates adjusting for competing risks.
The cytogenetic groups were compared using proportional hazards regression models for overall mortality (1- overall survival), relapse and TRM. The proportional hazards assumptions for all the variables were examined by adding a time-dependent covariate as necessary. Time dependent covariates with piecewise constant of regression coefficients were used to model time-varying effect when the proportionality assumption did not hold with the optimal time cut point determined by the maximum likelihood method. The proportionality assumption was further examined for the piecewise constant regression coefficient Cox model. A forward stepwise method was used to build the regression model for the outcomes of relapse, TRM and overall mortality. Since the cytogenetic groups were the main interest of this study, this variable was included in all steps of model building procedure with other covariates retained as indicated. Risk factors with significance level of p < 0.05 were included in the model. The potential interaction between main effect of cytogenetic group and all significant covariates was examined. For the subset analysis focus on chromosome 7 abnormalities, the same models were built with the main effect modified. Adjusted probabilities of LFS and OS were computed based on final Cox regression model, stratified by status groups, and weighted by the pooled sample proportion value for all significant risk factors. These adjusted probabilities estimate likelihood of outcomes in populations with similar prognostic factors. SAS version 9.2 was used in all analyses.
Results
Demographics
Tables 1a and b outline the demographics of patients with AML and MDS cohorts, respectively. Patients with MK+ AML were generally older than IRabn and other unfavorable cohorts, but similar to patients with normal karyotype. Leukocyte count at diagnosis was lower for MK+AML than the other groups. The proportion of patients with extra medullary disease or therapy-related AML was similar across the groups. There were a higher proportion of patients with <90% KPS and recipients of RIC regimens in the MK+ AML cohort. Additionally, peripheral blood stem cells (PBSC) was the predominant graft source for patients with MK+ AML and normal karyotype. The time from diagnosis to transplant, year of transplant, GVHD prophylaxis, use of growth factor support and in vivo T-cell depletion were similar across the AML cytogenetic groups. Among patients with MDS, MK+MDS and patients with normal karyotype were older than the other groups. The MK+MDS group had more patients with performance score less than 90%, and both MK+MDS and MDS- IRabn had a higher proportion of patients with pre-HCT marrow blasts between 11–20%. High International Prognostic Staging System (IPSS) was mainly observed in patients with MK+MDS and other unfavorable groups due to the cytogenetic component of the score. Patients with MK+MDS had a shorter time from diagnosis to transplant than others. Greater than 70% of patients in both unfavorable cytogenetic groups had evidence of abnormalities of chromosome 7. Similar to AML, most patients with MDS received PBSC as the graft source. Other variables including year of transplant, conditioning regimen intensity, GVHD prophylaxis, use of growth factor support and in vivo T-cell depletion were similar across the MDS groups.
Table 1a.
Demographic data on hematopoietic cell transplant recipients with AML from 1998–2011 according to cytogenetic groups.
| Variable | MK positive | Other unfavorable | Intermediate | Normal |
|---|---|---|---|---|
| Number of patients | 240 | 1138 | 1579 | 643 |
| Number of centers | 80 | 192 | 224 | 138 |
| Age at transplant, median (years) | 53 (1–75) | 43 (1–76) | 43 (<1–74) | 52 (2–74) |
| Age at transplant, years | ||||
| 0–20 | 13 (5) | 192 (17) | 255 (16) | 49 (8) |
| 21–40 | 45 (19) | 302 (27) | 432 (27) | 107 (17) |
| 41–60 | 123 (51) | 496 (44) | 738 (47) | 332 (52) |
| 61–64 | 30 (13) | 94 (8) | 97 (6) | 87 (14) |
| >= 65 | 29 (12) | 54 (5) | 57 (4) | 68 (11) |
| Gender | ||||
| Male | 146 (61) | 572 (50) | 816 (52) | 324 (50) |
| Female | 94 (39) | 566 (50) | 763 (48) | 319 (50) |
| Performance Score | ||||
| 90–100% | 134 (56) | 798 (70) | 1186 (75) | 444 (69) |
| < 90% | 90 (38) | 293 (26) | 338 (21) | 185 (29) |
| Missing | 16 (7) | 47 (4) | 55 (3) | 14 (2) |
| Type of AML | ||||
| De novo | 168 (70) | 848 (75) | 1256 (80) | 454 (71) |
| Secondary | 71 (30) | 288 (25) | 316 (20) | 188 (29) |
| Unknown | 1 (<1) | 2 (<1) | 7 (<1) | 1 (<1) |
| Number of cycles to achieve CR | ||||
| 1 | 67 (28) | 385 (34) | 785 (50) | 240 (37) |
| > 1 | 65 (27) | 260 (23) | 386 (24) | 176 (27) |
| Unknown | 108 (45) | 493 (43) | 408 (26) | 227 (35) |
| Extra medullary disease | ||||
| No | 227 (95) | 1058 (93) | 1446 (92) | 584 (91) |
| Yes | 13 (5) | 80 (7) | 133 (8) | 59 (9) |
| White blood cell at diagnosis (median, ×10ˆ9/L) | 3 (<1–53) | 6 (<1–224) | 9 (<1–118) | 10 (<1–260) |
| Cytogenetic score | ||||
| Normal | 0 | 0 | 0 | 643 |
| Intermediate | 0 | 0 | 1579 | 0 |
| Poor | 240 | 1138 | 0 | 0 |
| MK status | ||||
| MK+: more than 1 monosomy | 136 (57) | 0 | 0 | 0 |
| MK+: 1 monosomy + other | 104 (43) | 0 | 0 | 0 |
| Other | 0 | 1138 | 1578 | 643 |
| Abnormality −7/7q | ||||
| No | 92 (38) | 863 (76) | 1579 | 643 |
| Yes | 148 (62) | 275 (24) | 0 | 0 |
| Conditioning regimen | ||||
| TBI + Cy +- others | 48 (20) | 320 (28) | 392 (25) | 156 (24) |
| TBI +- others | 28 (12) | 72 (6) | 156 (10) | 62 (10) |
| Bu + Cy +- others | 57 (24) | 389 (34) | 611 (39) | 147 (23) |
| Bu + Flud +- others | 73 (30) | 236 (21) | 278 (18) | 217 (34) |
| Flud + Mel +- others | 21 (9) | 77 (7) | 74 (5) | 34 (5) |
| Other conditioning regimen | 13 (5) | 44 (4) | 68 (4) | 27 (4) |
| Conditioning regimen intensity | ||||
| Myeloablative | 139 (58) | 857 (75) | 1163 (74) | 440 (68) |
| RIC | 101 (42) | 281 (25) | 416 (26) | 203 (32) |
| Time from diagnosis to transplant, median (months) | 5 (1–40) | 5 (1–118) | 5 (1–165) | 5 (1–91) |
| Time from diagnosis to transplant | ||||
| 0–3 months | 17 (7) | 106 (9) | 188 (12) | 63 (10) |
| 3–6 months | 159 (66) | 723 (64) | 899 (57) | 385 (60) |
| >= 6 months | 64 (27) | 309 (27) | 492 (31) | 195 (30) |
| Type of donor | ||||
| HLA-identical sibling | 75 (31) | 481 (42) | 1028 (65) | 289 (45) |
| Unrelated 8/8 | 122 (51) | 499 (44) | 387 (25) | 268 (42) |
| Unrelated 7/8 | 43 (18) | 158 (14) | 164 (10) | 86 (13) |
| Missing | 11 (5) | 42 (4) | 61 (4) | 21 (3) |
| Graft type | ||||
| Bone marrow | 46 (19) | 359 (32) | 523 (33) | 126 (20) |
| Peripheral blood | 194 (81) | 779 (68) | 1056 (67) | 517 (80) |
| GVHD prophylaxis | ||||
| CNI plus Methotrexate | 162 (68) | 797 (70) | 1162 (74) | 439 (68) |
| CNI plus MMF | 54 (23) | 190 (17) | 209 (13) | 135 (21) |
| CNI +- others | 20 (8) | 125 (11) | 167 (11) | 60 (9) |
| Other | 4 (2) | 26 (2) | 41 (3) | 9 (1) |
| ATG/Alemtuzumab | 68 (28) | 308 (28) | 331 (21) | 178 (28) |
| Planned GM or GCSF (<12 days post HCT) | 96 (40) | 472 (41) | 694 (44) | 239 (37) |
|
| ||||
| Median follow-up of survivors (range), months | 49 (4–144) | 60 (3–171) | 70 (3–172) | 37 (3–122) |
Abbreviations: AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; Bu, busulfan; CNI, calcineurin inhibitor; Cy, cyclophosphamide; Flud, fludarabine; GM/GCSF, granulocyte and macrophage or granulocyte growth factor; MK, monosomal karyotype; MMF, micophenolate mofetil; Mel; melphalan; RIC, reduced intensity conditioning; TBI, total body irradiation.
Table 1b.
Demographic data on hematopoietic cell transplant recipients with MDS from 1998–2011 according to cytogenetic groups.
| Variable | MK positive | Other unfavorable | Intermediate | Normal | Favorable |
|---|---|---|---|---|---|
| Number of patients | 221 | 423 | 611 | 241 | 98 |
| Number of centers | 85 | 138 | 162 | 78 | 56 |
| Age at transplant, median (years) | 56 (8–74) | 49 (<1–74) | 50 (1–74) | 57 (4–72) | 54 (12–72) |
| Age at transplant, years | |||||
| 0–20 | 10 (5) | 67 (16) | 58 (9) | 8 (3) | 2 (2) |
| 21–40 | 26 (12) | 76 (18) | 122 (20) | 26 (11) | 10 (10) |
| 41–60 | 123 (56) | 189 (45) | 314 (51) | 117 (49) | 57 (58) |
| 61–64 | 33 (15) | 58 (14) | 80 (13) | 55 (23) | 15 (15) |
| >= 65 | 29 (13) | 33 (8) | 37 (6) | 35 (15) | 14 (14) |
| Gender | |||||
| Male | 128 (58) | 247 (58) | 361 (59) | 148 (61) | 62 (63) |
| Female | 93 (42) | 176 (42) | 250 (41) | 93 (39) | 36 (37) |
| Performance Score | |||||
| 90–100% | 120 (54) | 300 (71) | 405 (66) | 161 (67) | 55 (56) |
| < 90% | 91 (41) | 104 (25) | 182 (30) | 70 (29) | 37 (38) |
| Missing | 10 (5) | 19 (4) | 24 (4) | 10 (4) | 6 (6) |
| IPSS prior to transplant | |||||
| Low | 0 | 0 | 0 | 101 (42) | 21 (21) |
| Intermediate-1 | 63 (29) | 133 (31) | 400 (65) | 118 (49) | 61 (62) |
| Intermediate-2 | 119 (54) | 202 (48) | 147 (24) | 7 (3) | 8 (8) |
| High | 21 (10) | 29 (7) | 1 (<1) | 0 | 0 |
| Missing | 18 (8) | 59 (14) | 63 (10) | 15 (6) | 8 (8) |
| Bone marrow blasts prior to transplant | |||||
| < 5% | 117 (53) | 233 (55) | 329 (54) | 148 (61) | 56 (57) |
| 5–10% | 59 (27) | 99 (23) | 155 (25) | 67 (28) | 26 (27) |
| 11–20% | 28 (13) | 36 (9) | 67 (11) | 14 (6) | 8 (8) |
| > 20% | 0 | 6 (1) | 4 (<1) | 1 (<1) | 1 (1) |
| Missing | 17 (8) | 49 (12) | 56 (9) | 11 (5) | 7 (7) |
| Disease status at transplant | |||||
| Early | 71 (32) | 183 (43) | 266 (44) | 82 (34) | 50 (51) |
| Advanced | 150 (68) | 240 (57) | 345 (56) | 159 (66) | 48 (49) |
| Cytogenetic group | |||||
| Normal | 0 | 0 | 0 | 241 | 0 |
| Favorable | 1 (<1) | 0 | 0 | 0 | 98 |
| Intermediate | 6 (3) | 0 | 611 | 0 | 0 |
| Poor | 214 (97) | 423 | 0 | 0 | 0 |
| Abnormality −7/7q | |||||
| No | 50 (23) | 119 (28) | 611 | 241 | 98 |
| Yes | 171 (77) | 304 (72) | 0 | 0 | 0 |
| Conditioning regimen | |||||
| TBI + Cy +- others | 21 (10) | 80 (19) | 100 (16) | 24 (10) | 12 (12) |
| TBI +- others | 22 (10) | 40 (9) | 61 (10) | 25 (10) | 4 (4) |
| Bu + Cy +- others | 62 (28) | 126 (30) | 225 (37) | 67 (28) | 27 (28) |
| Bu + Flud +- others | 85 (38) | 110 (26) | 127 (21) | 92 (38) | 39 (40) |
| Flud + Mel +- others | 20 (9) | 45 (11) | 57 (9) | 19 (8) | 15 (15) |
| Other conditioning regimen | 11 (5) | 22 (5) | 41 (7) | 14 (6) | 1 (1) |
| Conditioning regimen intensity | |||||
| Myeloablative | 140 (63) | 265 (63) | 376 (62) | 140 (58) | 55 (56) |
| RIC | 81 (37) | 158 (37) | 235 (38) | 101 (42) | 43 (44) |
| Time from diagnosis to transplant, median (months) | 5 (1–187) | 6 (<1–131) | 8 (1–275) | 9 (1–284) | 12 (1–266) |
| Time from diagnosis to transplant | |||||
| 0–3 months | 22 (10) | 50 (12) | 59 (10) | 14 (6) | 5 (5) |
| 3–6 months | 104 (47) | 162 (38) | 161 (26) | 57 (24) | 19 (19) |
| >= 6 months | 95 (43) | 211 (50) | 391 (64) | 170 (71) | 74 (76) |
| Type of donor | |||||
| HLA-identical sibling | 69 (31) | 132 (31) | 331 (54) | 100 (41) | 36 (37) |
| Unrelated 8/8 | 122 (55) | 215 (51) | 204 (33) | 122 (51) | 49 (50) |
| Unrelated 7/8 | 30 (14) | 76 (18) | 76 (12) | 19 (8) | 13 (13) |
| Graft type | |||||
| Bone marrow | 48 (22) | 144 (34) | 184 (30) | 46 (19) | 24 (24) |
| Peripheral blood | 173 (78) | 279 (66) | 427 (70) | 195 (81) | 74 (76) |
| GVHD prophylaxis | |||||
| CNI plus Methotrexate | 138 (62) | 278 (66) | 396 (65) | 149 (62) | 66 (67) |
| CNI plus MMF | 56 (25) | 93 (22) | 109 (18) | 61 (25) | 15 (15) |
| CNI +- others | 18 (8) | 39 (9) | 95 (16) | 25 (10) | 17 (17) |
| Other | 9 (4) | 13 (3) | 11 (2) | 6 (2) | 0 |
| ATG/Campath | 89 (40) | 139 (33) | 171 (28) | 80 (33) | 29 (30) |
| Planned GM or GCSF (<12 days post HCT) | 99 (45) | 185 (44) | 274 (45) | 104 (43) | 43 (44) |
| Median follow-up of survivors (range), months | 35 (10–96) | 53 (3–171) | 53 (3–174) | 36 (6–72) | 60 (4–167) |
Abbreviations: AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; Bu, busulfan; CNI, calcineurin inhibitor; Cy, cyclophosphamide; Flud, fludarabine; GM/GCSF, granulocyte and macrophage or granulocyte growth factor; MK, monosomal karyotype; MMF, micophenolate mofetil; Mel; melphalan; RIC, reduced intensity conditioning; TBI, total body irradiation.
Disease Relapse
Three-year cumulative incidences of leukemia relapse were 52% (95% confidence interval [CI], 42–58%), 36 % (95% CI, 34–39%), 25% (95% CI, 23–27%) and 30 (95% CI, 26–34%) for MK+ AML, other unfavorable, IRabn and normal karyotype, respectively (p<0.001) (Figure 1a). Multivariate analysis of leukemia relapse demonstrated that MK+AML was associated with higher relapses compared to normal karyotype (relative risk [RR] 1.98, 95% CI 1.58–2.49, p<0.001) (Table 2), to IRabn (RR 2.20, 95% CI 1.78–2.72, p<0.001) and to other unfavorable (RR 1.46, 95% CI 1.19–1.79, p<0.001). AML with other unfavorable cytogenetics was associated with higher relapse risk compared to normal karyotype (RR 1.36, 95% CI 1.14–1.63, p<0.001) and to IRabn (RR 1.51, 95% CI 1.32–1.74, p<0.001). Other variables associated with higher rates of leukemia relapse include older age and reduced conditioning intensity, lower performance score and graft source (Appendix, Table A). Older patients receiving RIC experienced higher disease relapses compared to younger patients receiving MA conditioning. Among patients age 41–60 years, those who received a MA regimen had lower relapse risks than recipients of RIC (RR 0.58 95% CI, 0.49–0.69, p<0.001). Additionally recipients of PBSC experienced lower rates of relapse compared to bone marrow recipients (RR 0.84 95% CI, 0.73–0.98, p=0.02). For MDS patients, the 3-year cumulative incidence of relapse were 44% (95% CI, 37–51%), 32% (95% CI, 27–36%), 26% (95% CI, 23–30%), 28% (95% CI, 22–34%) and 29% (95% CI, 20–39%) for MK+ AML, other unfavorable, IRabn, normal karyotype and favorable groups, respectively (p<0.001) (Figure 1b). Multivariate analysis of MDS relapse demonstrated that MK+MDS was associated with higher relapses compared to normal karyotype (RR 2.39, 95% CI 1.74–3.29, p<0.001)(Table 2), to IRabn (RR 2.13, 95% CI 1.64–2.76, p<0.001), to other unfavorable (RR 1.59, 95% CI 1.21–2.09, p<0.001) and to favorable (RR 2.01 95% CI, 1.32–3.06, p=001). Other variables associated with higher rates of MDS relapse include older age/RIC, lower performance score, BM grafts, ATG or Alemtuzumab, no planned use of growth factor and advanced disease status at transplant (Appendix Table B). Younger patients and recipients of MA experienced lower relapse rates. Among patients age 41–60 years MA conditioning led to lower relapse risks than RIC/NMA (RR 0.67 95% CI, 0.51–0.90, p=0.007). The planned use of growth factor was associated with lower relapse rates in MDS (RR 0.79 95% CI, 0.66–0.95, p=0.01).
Figure 1.
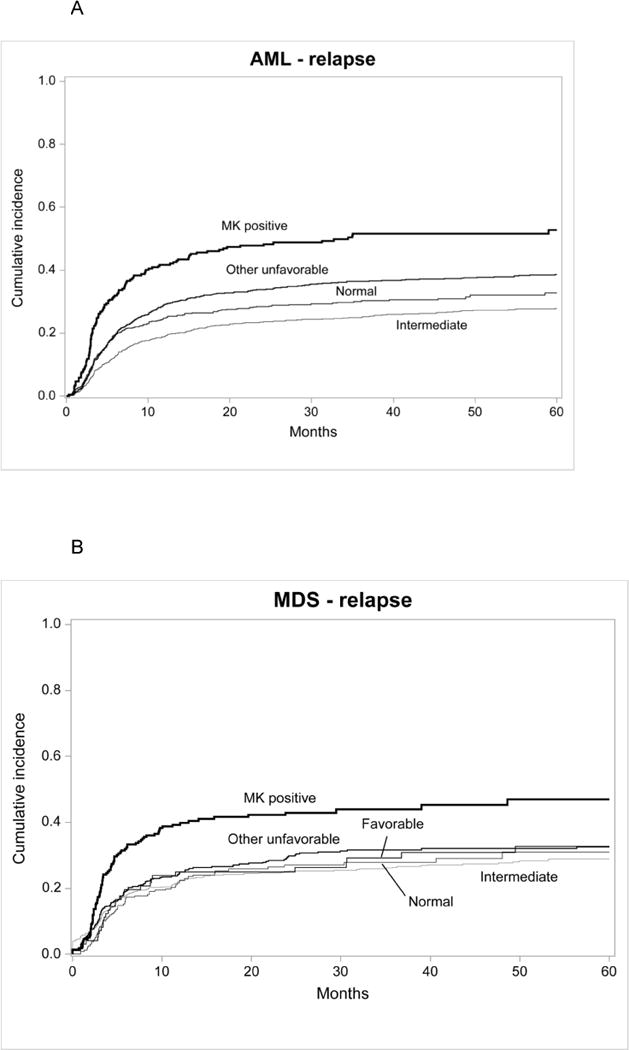
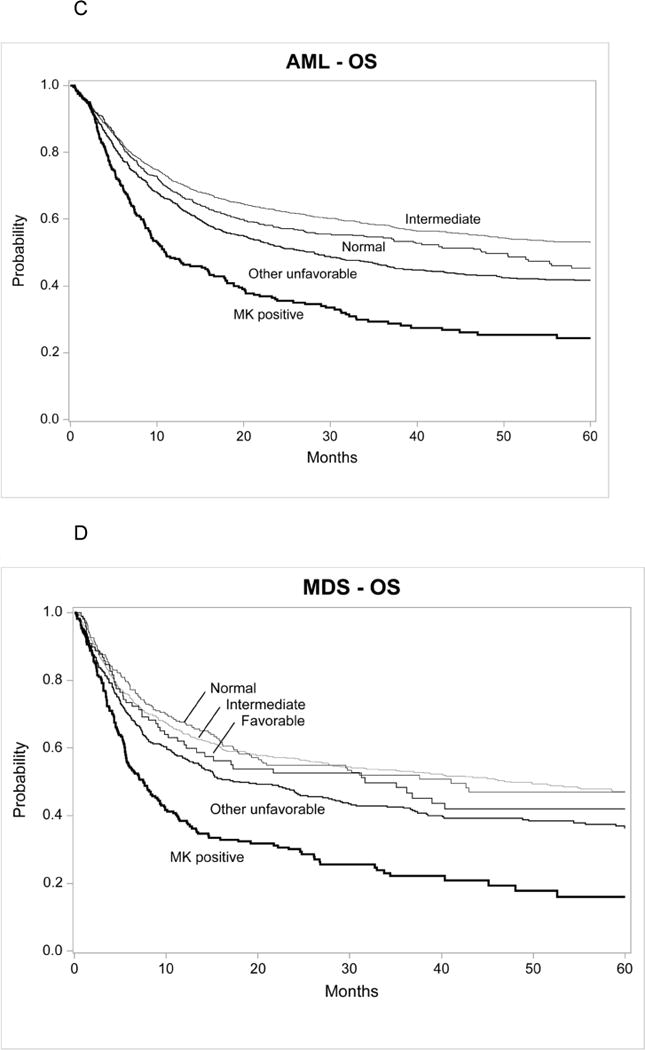
Cumulative incidence of disease relapse for AML in first complete remission(1A) and MDS (1B) and overall survival for AML in first complete remission (1C) and MDS (1D) after HCT,
Table 2.
Multivariate analysis of treatment related mortality, relapse, treatment failure (1-LFS) and overall mortality for AML and MDS by cytogenetic groups and adjusted for significant covariates.
| AML | N | Relative Risk | P-value | MDS | N | Relative Risk | P-value |
|---|---|---|---|---|---|---|---|
| TRM | TRM | ||||||
| Normal | 641 | 1.00a | <0.411 | Normal | 237 | 1.00a | < 0.0011 |
| MK positive | 238 | 1.01 (0.74–1.39) | 0.94 | MK positive | 219 | 1.80 (1.27–2.54) | < 0.001 |
| Other unfavorable | 1133 | 0.95 (0.77–1.18) | 0.66 | Other unfavorable | 416 | 1.37 (0.99–1.90) | 0.06 |
| Intermediate | 1568 | 0.86 (0.70–1.06) | 0.16 | Intermediate | 606 | 1.01 (0.73–1.39) | 0.97 |
| Favorable | 97 | 0.95 (0.59–1.52) | 0.83 | ||||
| Relapse | Relapse | ||||||
| Normal | 641 | 1.00a | < 0.0011 | Normal | 237 | 1.00a | < 0.0011 |
| MK positive | 238 | 1.98 (1.58–2.49) | < 0.001 | MK positive | 219 | 2.39 (1.74–3.29) | < 0.001 |
| Other unfavorable | 1133 | 1.36 (1.14–1.63) | < 0.001 | Other unfavorable | 416 | 1.50 (1.11–2.04) | 0.009 |
| Intermediate | 1568 | 0.90 (0.75–1.08) | 0.27 | Intermediate | 606 | 1.12 (0.84–1.51) | 0.44 |
| Favorable | 97 | 1.19 (0.76–1.86) | 0.44 | ||||
| Treatment Failure: | Treatment Failure: | ||||||
| Normal | 641 | 1.00a | < 0.0011 | Normal | 237 | 1.00a | < 0.0011 |
| MK positive | 238 | 1.55 (1.29–1.86) | < 0.001 | MK positive | 219 | 2.17 (1.72–2.74) | < 0.001 |
| Other unfavorable | 1133 | 1.19 (1.04–1.37) | 0.01 | Other unfavorable | 416 | 1.52 (1.22–1.89) | < 0.001 |
| Intermediate | 1568 | 0.88 (0.77–1.02) | 0.09 | Intermediate | 606 | 1.13 (0.92–1.40) | 0.25 |
| Favorable | 97 | 1.15 (0.84–1.57) | 0.40 | ||||
| Overall Mortality: | Overall Mortality: | ||||||
| Normal | 643 | 1.00a | < 0.0011 | Normal | 241 | 1.00a | < 0.0011 |
| MK positive | 240 | 1.67 (1.38–2.01) | < 0.001 | MK positive | 221 | 2.02 (1.59–2.59) | < 0.001 |
| Other unfavorable | 1138 | 1.22 (1.06–1.40) | 0.006 | Other unfavorable | 423 | 1.39 (1.10–1.77) | 0.006 |
| Intermediate | 1579 | 0.90 (0.78–1.05) | 0.17 | Intermediate | 611 | 0.96 (0.76–1.22) | 0.73 |
| Favorable | 98 | 1.00 (0.71–1.41) | 1.00 |
Overall P value
Reference group
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MK, monosomal karyotype; TRM, transplant related mortality
Transplant Related Mortality
For AML, the 3-year cumulative incidences of TRM were 22% (95% CI, 17–27%), 22 % (95% CI, 19–24%), 20% (95% CI, 18–22%) and 20 (95% CI, 17–23%) for MK+ AML, other unfavorable, IRabn and normal karyotype, respectively (p=0.75). Multivariate analysis showed no impact of cytogenetic abnormalities on TRM for AML (p=0.41). Other variables associated with TRM were age/conditioning intensity, lower performance score, conditioning regimen type, unrelated and HLA mismatched donor, PBSC grafts, GVHD prophylaxis, planned use of growth factors and year of transplant (Appendix Table C).
For MDS the 3-year cumulative incidences of TRM were 37% (95% CI, 30–44%), 32% (95% CI, 27–37%), 27% (95% CI, 24–31%), 26% (95% CI, 20–32%) and 28 (95% CI, 19–38%) for MK+ MDS, other unfavorable, IRabn, normal karyotype and favorable, respectively (p=0.07). Multivariate analysis of TRM in MDS showed that MK+MDS was associated with higher TRM compared to normal karyotype (RR 1.80, 95% CI 1.27–2.54, p<0.001)(Table 2), to IRabn (RR 1.79, 95% CI 1.34–2.38, p<0.001), to other unfavorable (RR 1.31, 95% CI 0.98–1.76, p=0.07) and to favorable (RR 1.89 95% CI, 1.22–2.94, p=0.005). Other variables associated with higher rates of TRM in MDS include older age/conditioning intensity, lower performance score, unrelated 7/8 HLA matched donor, advanced disease status and year of transplant (Appendix Table D).
Graft-versus-Host Disease
Cumulative incidences of grades II–IV acute GVHD at day 100 among patients with AML were 43% (95% CI, 37–49%), 35% (95% CI, 32–38%), 30% (95% CI, 28–32%) and 33% (95% CI, 30–37%) for MK+ AML, other unfavorable, IRabn and normal karyotype, respectively (p<0.01). Cumulative incidences of chronic GVHD at 1 year among patients with AML were 44% (95% CI, 37–50%), 43% (95% CI, 40–46%), 44% (95% CI, 42–47%) and 48% (95% CI, 44–52%) for MK+ AML, other unfavorable, IRabn and normal karyotype, respectively (p=0.26).
Cumulative incidences of grades II–IV acute GVHD at day 100 among patients with MDS were 48% (95% CI, 41–54%), 45% (95% CI, 40–50%), 39% (95% CI, 35–42%), 38% (95% CI, 31–44%) and 42 (95% CI, 32–51%) for MK+ MDS, other unfavorable, IRabn, normal karyotype and favorable, respectively (p=0.03). Cumulative incidences of chronic GVHD at 1 year among patients with MDS were 39% (95% CI, 33–46%), 25% (95% CI, 21–29%), 23% (95% CI, 19–26%), 22% (95% CI, 17–28%) and 25% (95% CI, 17–34%) for MK+ MDS, other unfavorable, IRabn, normal karyotype and favorable, respectively (p=0.03).
Disease Free Survival and Overall Survival
Three-year probabilities of DFS in AML were 27% (95% CI, 21–33%), 42% (95% CI, 39–45%), 55% (95% CI, 52–58%) and 50 (46–54%) for MK+ AML, other unfavorable, IRabn and normal karyotype, respectively (p<0.001). Corresponding three-year probabilities for overall survival in AML were 29% (95% CI, 24–35%), 46% (95% CI, 43–49%), 58% (95% CI, 56–61%) and 55 (51–59%), respectively (p<0.001) (Figure 1c). Multivariate analysis of overall mortality demonstrated that MK+AML was associated with higher mortality compared to normal karyotype (RR 1.67, 95% CI 1.38–2.01, p<0.001) (Table 2), to IRabn (RR 1.84, 95% CI 1.55–2.19, p<0.001) and to other unfavorable (RR 1.37, 95% CI 1.15–1.62, p<0.001). AML with other unfavorable was associated with higher mortality compared to normal karyotype (RR 1.22, 95% CI 1.06–1.40, p<0.001) and to IRabn (RR 1.35, 95% CI 1.20–1.50, p<0.001). Other variables associated with higher rates of leukemia relapse include older age/conditioning intensity, lower performance score, unrelated or HLA mismatched donor and year of transplant (Appendix Table E). Older patients receiving RIC were associated with higher mortality compared to younger patients receiving myeloablative conditioning. Among patients age 41–60 years a MA regimen led to lower mortality than RIC (RR 0.77 95% CI, 0.67–0.89, p<0.001).
Three-year probabilities of DFS in MDS were 19% (95% CI, 13–25%), 36% (95% CI, 32–41%), 46% (95% CI, 42–50%), 46% (95% CI, 40–53%) and 42% (95% CI, 32–53%) for MK+ MDS, other unfavorable, IRabn, normal karyotype and favorable, respectively (p<0.01). Corresponding three-year probabilities for overall survival in MDS were 22% (95% CI, 16–29%), 42% (95% CI, 37–47%), 53% (95% CI, 49–57%), 52% (95% CI, 45–59%) and 48% (95% CI, 38–59%) for MK+ MDS, other unfavorable, IRabn, normal karyotype and favorable, respectively (p<0.01) (Figure 1d). Multivariate analysis of overall mortality demonstrated that MK+MDS was associated with higher mortality compared to normal karyotype (RR 2.02, 95% CI 1.59–2.59, p<0.001)(Table 2), to IRabn (RR 2.11, 95% CI 1.73–2.58, p<0.001), to other unfavorable (RR 1.45, 95% CI 1.19–1.78, p<0.001) and to favorable (RR 2.02 95% CI, 1.22–2.78, p=001). MDS with other unfavorable was associated with higher mortality compared to normal karyotype (RR 1.39, 95% CI 1.10–1.77, p=0.006), to IRabn (RR 1.45, 95% CI 1.22–1.72, p<0.001) and to favorable (RR 1.39, 95% CI 1.03–1.88, p=0.03). Other variables associated with higher mortality in MDS include older age/conditioning intensity, lower performance score, unrelated 7/8 HLA matched donor, advanced disease status and year of transplant (Appendix Table F). Younger patients and recipients of MA experienced better survival. Among patients age 41–60, MA conditioning led to similar survival as RIC (RR 0.95 95% CI, 0.79–1.15, p=0.62).
Chromosome 7 Abnormalities Subset Analyses
For this subset, both the AML and MDS cohorts were stratified into three groups: MK+ with abnormal −7/−7q (MK+/abn7, AML N=148, MDS N=171), abnormal −7/−7q without MK (AML N=275, MDS N=304) and normal karyotype (AML N=643, MDS N=241). The demographic differences across these groups were similar to those in the whole population for AML and MDS. Among patients with AML, there was higher relapse and worse survival for patients with MK+/7Abn (Figure 2a). Multivariate analysis confirmed a higher mortality with MK+/7abn compared to normal karyotype (RR 1.98 95% CI, 1.58–2.46, p<0.001) and abn7 without MK+ (RR 1.72 95% CI, 1.34–2.20, p<0.001). Among patients with MDS, patients with MK+/7abn experienced higher TRM, more relapse and worse survival (Figure 2b). Multivariate analysis among patients with MDS confirmed higher mortality with MK+/7abn compared to normal karyotype (RR 2.06 95% CI, 1.58–2.68, p<0.001) and abn7 without MK+ (RR 1.79 95% CI, 1.39–2.32, p<0.001).
Figure 2.
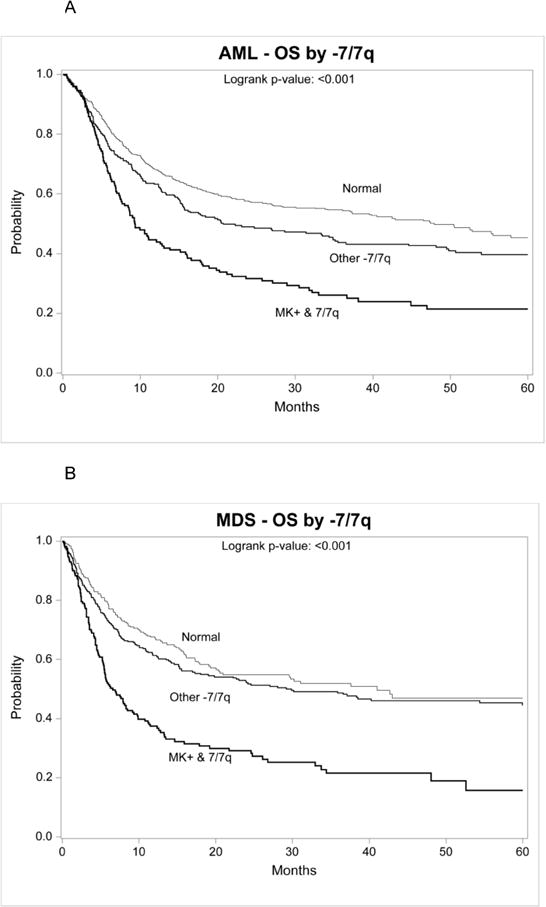
Overall survival for AML in first complete remission (2A) and MDS (2B) after HCT defined as chromosome 7 abnormalities with or without monosomal karyotype (MK+) and normal karyotype
Discussion
This large analysis of patients with MK+ AML in CR1 and MDS who received an allogeneic HCT confirms the finding of higher risks of relapse and significantly worse post-HCT outcomes compared to other cytogenetically defined groups including other previously defined unfavorable groups. The worse survival in MK+AML was mainly driven by excess in relapse, whereas in MK+MDS led to excess risks of both TRM and relapse. We also explored the conditioning regimen effect within the cytogenetically defined groups. Generally, younger patients who received a MA regimen had better outcomes in AML. Among patients within 40–61 years, MA resulted in better survival than RIC for AML but not in MDS (Appendix Tables). However, the adverse prognostic impact of MK+ disease was not overcome by conditioning intensity and we observed no significant interactions between these two variables.
The incidence of MK+AML is reported in 11 to 13% of patients with AML and approximately 30% in patients with AML with abnormal cytogenetics (3, 12, 13, 20). MK+ AML patients are generally older age, with low leukocyte count at diagnosis and more often have complex cytogenetics as observed in this study. Medeiros et al analyzed a large series from patients with AML enrolled in upfront clinical trials in the US and reported a 20% incidence of MK+AML in patients older than 60 years. Kayser et al in a series of 319 patients with MK+ AML from the German-Austrian AML Study Group also observed MK+ patients to be older with lower leukocyte count at diagnosis and associated with abnormalities of chromosomes 7, 5, 17p, 18q, 20q, 3 and complex karyotype (20). Interestingly, patients with MK+AML present less frequently with commonly observed molecular markers such as FLT3 internal tandem duplication, NMP-1 mutation and tyrosine kinase domain mutations (20).
MK+ is closely related to complex cytogenetics, and as initially defined by Breems et al, MK+ represents a subset of the unfavorable risk with exceptionally poor outcomes(3). Complex cytogenetics is a general definition with a number of cytogenetic abnormalities, 3–5 or greater(2). The prognosis with MK+ is worse than complex cytogenetics, likely related the higher proportion of TP53 deletion seen in MK+AML (20–23). However, patients with many cytogenetic abnormalities most often also meet the criteria for MK+. Thus for MK+ there is general loss of chromosomes and complex cytogenetics without MK+ includes a hyperdiploid karyotype. Additionally, most poor risk single karyotypic abnormalities in AML include loss of chromosome 5/5q, 7/7q, 12p, 17p, 18/18q and 20 which are correlated with MK+ (12, 24–27). Phenotypic analysis of leukemic blasts demonstrated that co-expression of monocytic marker CD11b to be independently associated with poor outcomes and closely related with MK+ and older age at diagnosis(28). Analysis of multidrug resistant (MDR) functional activity among 23 patients with MK+AML demonstrated a high frequency of MDR compared to other AML subgroups and helps explain the aggressive behavior (29). Another association of MK+AML is mutations in the tumor-suppressor gene neurofibromatosis-1 (NF-1) manifested through somatic deletions of 17q11 (30). NF-1 mutations present in AML are also associated with poor outcomes. MK+ appears to be a surrogate marker for genomic instability in AML subclones where the absence of important tumor suppressor and cell cycle checkpoint genes helps confer a survival and proliferative advantage over other subclones.
MK+ AML yields low rates (only 20 to 30%) of CR and short remission duration yielding reported median survival of 8 to 10 months with 2 year survival less than 10% (3, 13, 20, 31, 32). The current HCT study includes only those achieving CR after induction therapy. Despite worse outcomes compared to other cytogenetic groups, the overall survival for MK+AML is 29% at 3 years, substantially better than reported without transplant. In fact, Cornelissen et al compared post remission therapies among 107 patients with MK+AML who received either an allogeneic HCT (N=45), autologous HCT or chemotherapy consolidation(33). Five-year overall survival after an allogeneic HCT was 19% versus only 8% with other therapy. Multivariate analysis demonstrated a 70% reduction of relapse with an allogeneic transplant.
Following HCT, Armand et al analyzed a large cohort from the CIBMTR to determine cytogenetic groups that would influence outcomes after HCT(34). This analysis separates patients in three groups identifying inv(16) and complex cytogenetics with >4 abnormalities as the extremes of favorable and unfavorable prognosis, respectively. MK+ AML has been consistently associated with high disease relapse and poor survival after an allogeneic HCT (14, 17, 20, 31, 33, 35–37). However many of MK+ patients are not eligible for MA regimens due to their age. RIC/NMA regimens is associated with higher rates of relapse, especially in patients with poor risk cytogenetics (10, 11). In the current analysis, for MK+ disease, even with MA regimens the outcomes were worse when compared to other cytogenetic groups.
Abnormalities with chromosome 7 were the most frequently observed and we observed that MK+ was prognostically worse than chromosome 7 abnormalities without MK+ (12, 37). The use of growth factors post transplant was tested in the current study because of reports that granulocyte colony-stimulating factor preferentially induces proliferation of cells with monosomy 7 (38). The early use of growth factor (planned to be given in the first 12 days of transplant) in AML was associated with higher TRM (RR 1.32, p<001) while in MDS it was associated with lower incidence of disease relapse (RR 0.79, p=0.01). The subset analyses focused on chromosome 7 abnormalities showed no further associations with growth factor use. G-CSF expression is increased in CD34+ cells with monosomy 7(38), which could theoretically may increase the risk of disease relapse. The relationship of growth factor used early in transplantation needs to be further evaluated related to timing and type of disease being treated.
MK+ MDS as a high risk subgroup is less well established, though MK+ and chromosome 7 abnormalities (20, 37) can also influence MDS outcomes. Cytogenetics is an integral component of the IPSS (4) and the new revised IPSS (5). The revised IPSS cytogenetics include very poor cytogenetics as complex (>3) cytogenetic abnormalities which are associated with MK+. Xing et al analyzed outcomes of MDS patients showing complex karyotype and MK+ yielding similar poor outcomes(39). The revised IPSS confirmed poor prognosis for the very poor cytogenetics category (40). MK+MDS after allogeneic HCT has been described with similar poor outcomes (41–44). The current analysis also demonstrated that MK+MDS were associated with higher TRM, in contrast to the models in AML in which the cytogenetic group had no impact on TRM. These results could possibly be explained by the fact that a larger proportion of patients with MK+MDS had intermediate-II or high IPSS compared to other groups, which would require more treatment prior to transplant than other MDS groups, although this is speculative. MK+ AML and MDS are high risk groups with disappointing survival, even after allogeneic transplant. Implementing interventions after transplant to further reduce disease relapse through additional targeted therapy (45) or by optimizing graft-versus leukemia are needed to improve outcomes.
Supplementary Material
The Highlights for this study include.
Patients with MK+ AML have worse survival after transplant compared to other AML in CR1.
MK+ in patients with MDS has a negative prognostic impact after allogeneic transplant.
The negative impact of MK+ is observed after myeloablative and reduced intensity conditioning
Acknowledgments
Additional members from the Writing Committee who offered input different stages of development of this study include: Camille N. Abboud, Bruce Camitta, William Drobyski, Sergio Giralt, Vincent Ho, Luis Isola, John Koreth, Mary Laughlin, Ian Lewis, Michael Lil, Selina Luger, Richard Maziarz, Ryan Mattinson, Joseph McGuirk Reinhold Munker, Amandee Salhorta, Salyka Sengsayadeth, Gerard Socié,
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Appendix Tables
Table A.
Multivariate analysis of relapse for AML, by monosomal karyotype
| Relative Risk | P-value | ||
|---|---|---|---|
| Main effect | |||
| Normal | 641 | 1.00a | Poverall < 0.001 |
| MK positive | 238 | 1.98 (1.58–2.49) | < 0.001 |
| Other unfavorable | 1133 | 1.36 (1.14–1.63) | < 0.001 |
| Intermediate | 1568 | 0.90 (0.75–1.08) | 0.27 |
| Other significant covariates: | |||
| Age at transplant by conditioning intensity, years | |||
| 0–20 MA | 470 | 1.00a | Poverall < 0.001 |
| 21–40 MA | 810 | 0.84 (0.68–1.04) | 0.11 |
| 41–60 MA | 1221 | 0.96 (0.78–1.19) | 0.73 |
| 41–60 RIC/NMA | 457 | 1.66 (1.31–2.10) | < 0.001 |
| 61–64 RIC/NMA | 241 | 1.65 (1.25–2.16) | < 0.001 |
| > 64 RIC/NMA | 187 | 1.82 (1.37–2.44) | < 0.001 |
| Others | 194 | 1.01 (0.73–1.38) | 0.97 |
| Karnofsky score | |||
| 90–100% | 2550 | 1.00a | Poverall = 0.006 |
| < 90% | 898 | 1.24 (1.09–1.42) | 0.001 |
| Missing | 132 | 1.10 (0.82–1.48) | 0.53 |
| Graft type | |||
| Bone marrow | 1046 | 1.00a | |
| Peripheral blood | 2534 | 0.84 (0.73–0.98) | 0.02 |
| Year of transplant | |||
| Continuous | 3580 | 1.04 (1.00–1.08) | 0.07 |
|
| |||
| Contrast | |||
| Main effect MK positive vs. other unfavorable | 1.46 (1.19–1.79) | < 0.001 | |
| Main effect MK positive vs. intermediate | 2.20 (1.78–2.72) | < 0.001 | |
| Main effect other unfavorable vs. intermediate | 1.51 (1.32–1.74) | < 0.001 | |
| Age 21–40 MA vs. 41–60 MA | 0.87 (0.73–1.03) | 0.11 | |
| Age 21–40 MA vs. 41–60 RIC/NMA | 0.50 (0.41–0.62) | < 0.001 | |
| Age 21–40 MA vs. 61–64 RIC/NMA | 0.51 (0.40–0.65) | < 0.001 | |
| Age 21–40 MA vs. > 64 RIC/NMA | 0.46 (0.35–0.60) | < 0.001 | |
| Age 21–40 MA vs. others | 0.83 (0.62–1.12) | 0.23 | |
| Age 41–60 MA vs. 41–60 RIC/NMA | 0.58 (0.49–0.69) | < 0.001 | |
| Age 41–60 MA vs. 61–64 RIC/NMA | 0.59 (0.47–0.73) | < 0.001 | |
| Age 41–60 MA vs. > 64 RIC/NMA | 0.53 (0.42–0.67) | < 0.001 | |
| Age 41–60 MA vs. others | 0.96 (0.72–1.27) | 0.77 | |
| Age 41–60 RIC/NMA vs. 61–64 RIC/NMA | 1.01 (0.79–1.29) | 0.94 | |
| Age 41–60 RIC/NMA vs. > 64 RIC/NMA | 0.91 (0.70–1.18) | 0.48 | |
| Age 41–60 RIC/NMA vs. others | 1.65 (1.22–2.23) | 0.001 | |
| Age 61–64 RIC/NMA vs. > 64 RIC/NMA | 0.90 (0.68–1.20) | 0.48 | |
| Age 61–64 RIC/NMA vs. others | 1.64 (1.17–2.28) | 0.004 | |
| Age > 64 RIC/NMA vs. others | 1.81 (1.29–2.55) | < 0.001 | |
| Karnofsky score < 90% vs. missing | 1.13 (0.83–1.54) | 0.43 | |
Reference group
Table B.
Multivariate analysis of relapse for MDS, by monosomal karyotype
| Relative Risk | P-value | ||
|---|---|---|---|
| Main effect: | |||
| Normal | 237 | 1.00a | Poverall < 0.001 |
| MK positive | 219 | 2.39 (1.74–3.29) | < 0.001 |
| Other unfavorable | 416 | 1.50 (1.11–2.04) | 0.009 |
| Intermediate | 606 | 1.12 (0.84–1.51) | 0.44 |
| Favorable | 97 | 1.19 (0.76–1.86) | 0.44 |
| Other significant covariates: | |||
| Age at transplant by conditioning intensity, years | |||
| 0–20 MA | 132 | 1.00a | Poverall < 0.001 |
| 21–40 MA | 210 | 1.04 (0.66–1.63) | 0.88 |
| 41–60 MA | 512 | 1.79 (1.20–2.67) | 0.004 |
| 41–60 RIC/NMA | 277 | 2.66 (1.69–4.18) | < 0.001 |
| 61–64 RIC/NMA | 158 | 2.29 (1.40–3.75) | 0.001 |
| > 64 RIC/NMA | 113 | 3.21 (1.89–5.43) | < 0.001 |
| Others | 173 | 1.88 (1.17–3.01) | 0.008 |
| Karnofsky score | |||
| 90–100% | 1032 | 1.00a | Poverall = 0.04 |
| < 90% | 476 | 1.27 (1.04–1.54) | 0.02 |
| Missing | 67 | 1.28 (0.85–1.92) | 0.24 |
| Conditioning regimen classification | |||
| TBI + Cy +- others | 233 | 1.00a | Poverall < 0.001 |
| TBI +- others | 150 | 0.84 (0.56–1.27) | 0.41 |
| Bu + Cy +- others | 506 | 0.86 (0.64–1.15) | 0.30 |
| Bu + Flud +- others | 446 | 1.02 (0.74–1.40) | 0.90 |
| Flud + Mel +- others | 153 | 0.37 (0.23–0.59) | < 0.001 |
| Other conditioning regimen | 87 | 1.29 (0.83–2.00) | 0.27 |
| Graft type | |||
| Bone marrow | 439 | 1.00a | |
| Peripheral blood | 1136 | 0.70 (0.56–0.87) | 0.002 |
| ATG/Alemtuzumab for conditioning or GVHD prophylaxis | |||
| ATG alone | 447 | 1.00a | Poverall = 0.005 |
| Alemtuzumab alone | 54 | 1.76 (1.15–2.69) | 0.009 |
| No ATG or Alemtuzumab | 1074 | 0.89 (0.72–1.09) | 0.25 |
| Planned GM or GCSF (12 days)b | |||
| No | 876 | 1.00a | |
| Yes | 699 | 0.79 (0.66–0.95) | 0.01 |
| Disease status at transplant | |||
| Early | 642 | 1.00a | |
| Advanced | 933 | 1.75 (1.44–2.12) | < 0.001 |
|
| |||
| Contrast | |||
| Main effect MK positive vs. other unfavorable | 1.59 (1.21–2.09) | < 0.001 | |
| Main effect MK positive vs. intermediate | 2.13 (1.64–2.76) | < 0.001 | |
| Main effect MK positive vs. favorable | 2.01 (1.32–3.06) | 0.001 | |
| Main effect other unfavorable vs. intermediate | 1.34 (1.06–1.69) | 0.01 | |
| Main effect other unfavorable vs. favorable | 1.26 (0.84–1.90) | 0.27 | |
| Main effect intermediate vs. favorable | 0.94 (0.63–1.41) | 0.78 | |
| Age 21–40 MA vs. 41–60 MA | 0.58 (0.41–0.81) | 0.001 | |
| Age 21–40 MA vs. 41–60 RIC/NMA | 0.39 (0.26–0.58) | < 0.001 | |
| Age 21–40 MA vs. 61–64 RIC/NMA | 0.45 (0.29–0.70) | < 0.001 | |
| Age 21–40 MA vs. > 64 RIC/NMA | 0.32 (0.20–0.52) | < 0.001 | |
| Age 21–40 MA vs. others | 0.55 (0.36–0.84) | 0.005 | |
| Age 41–60 MA vs. 41–60 RIC/NMA | 0.67 (0.51–0.90) | 0.007 | |
| Age 41–60 MA vs. 61–64 RIC/NMA | 0.78 (0.56–1.10) | 0.16 | |
| Age 41–60 MA vs. > 64 RIC/NMA | 0.56 (0.38–0.82) | 0.003 | |
| Age 41–60 MA vs. others | 0.95 (0.69–1.32) | 0.77 | |
| Age 41–60 RIC/NMA vs. 61–64 RIC/NMA | 1.16 (0.83–1.62) | 0.37 | |
| Age 41–60 RIC/NMA vs. > 64 RIC/NMA | 0.83 (0.57–1.20) | 0.32 | |
| Age 41–60 RIC/NMA vs. others | 1.42 (1.00–2.00) | 0.05 | |
| Age 61–64 RIC/NMA vs. > 64 RIC/NMA | 0.71 (0.47–1.08) | 0.11 | |
| Age 61–64 RIC/NMA vs. others | 1.22 (0.82–1.81) | 0.33 | |
| Age > 64 RIC/NMA vs. others | 1.71 (1.12–2.61) | 0.01 | |
| Karnofsky score < 90% vs. missing | 0.99 (0.65–1.51) | 0.97 | |
| Conditioning TBI +- others vs. Bu + Cy +- others | 0.98 (0.67–1.42) | 0.91 | |
| Conditioning TBI +- others vs. Bu + Flud +- others | 0.82 (0.58–1.16) | 0.26 | |
| Conditioning TBI +- others vs. Flud + Mel +- others | 2.28 (1.43–3.63) | < 0.001 | |
| Conditioning TBI +- others vs. other | 0.65 (0.42–1.01) | 0.06 | |
| Conditioning Bu + Cy +- others vs. Bu + Flud +- others | 0.84 (0.65–1.09) | 0.19 | |
| Conditioning Bu + Cy +- others vs. Flud + Mel +- others | 2.33 (1.49–3.63) | < 0.001 | |
| Conditioning Bu + Cy +- others vs. other | 0.67 (0.44–1.00) | 0.05 | |
| Conditioning Bu + Flud +- others vs. Flud+Mel+- others | 2.77 (1.85–4.16) | < 0.001 | |
| Conditioning Bu + Flud +- others vs. other | 0.79 (0.55–1.15) | 0.22 | |
| Conditioning Flud + Mel +- others vs. other | 0.29 (0.17–0.47) | < 0.001 | |
| Alemtuzumab alone vs. No ATG or Alemtuzumab | 1.98 (1.31–3.01) | 0.001 | |
Reference group
GF within 7d: RR=0.82, p<0.001
Table C.
Multivariate analysis of treatment-related mortality for AML, by monosomal karyotype
| N | Relative Risk | P-value | |
|---|---|---|---|
| Main effect: | |||
| Normal | 641 | 1.00a | Poverall = 0.41 |
| MK positive | 238 | 1.01 (0.74–1.39) | 0.94 |
| Other unfavorable | 1133 | 0.95 (0.77–1.18) | 0.66 |
| Intermediate | 1568 | 0.86 (0.70–1.06) | 0.16 |
| Other significant covariates: | |||
| Age at transplant by conditioning intensity, years | |||
| 0–20 MA | 470 | 1.00a | Poverall < 0.001 |
| 21–40 MA | 810 | 1.46 (1.06–2.02) | 0.02 |
| 41–60 MA | 1221 | 2.09 (1.53–2.83) | < 0.001 |
| 41–60 RIC/NMA | 457 | 2.03 (1.39–2.96) | < 0.001 |
| 61–64 RIC/NMA | 241 | 2.61 (1.72–3.94) | < 0.001 |
| > 64 RIC/NMA | 187 | 2.61 (1.69–4.03) | < 0.001 |
| Others | 194 | 1.88 (1.24–2.86) | 0.003 |
| Karnofsky score | 0.001 | ||
| 90–100% | 2550 | 1.00a | |
| < 90% | 898 | 1.32 (1.13–1.54) | Poverall < 0.001 |
| Missing | 132 | 1.31 (0.94–1.82) | 0.11 |
| Conditioning regimen classification | 0.04 | ||
| TBI + Cy +- others | 911 | 1.00a | |
| TBI +- others | 315 | 0.85 (0.63–1.16) | 0.31 |
| Bu + Cy +- others | 1198 | 0.83 (0.69–1.01) | 0.06 |
| Bu + Flud +- others | 802 | 0.72 (0.57–0.90) | 0.004 |
| Flud + Mel +- others | 203 | 0.95 (0.68–1.32) | 0.77 |
| Other conditioning regimen | 151 | 0.68 (0.45–1.02) | 0.06 |
| HLA matching | |||
| HLA-identical sibling | 1864 | 1.00a | Poverall < 0.001 |
| Unrelated 8/8 | 1269 | 1.45 (1.23–1.71) | < 0.001 |
| Unrelated 7/8 | 447 | 2.13 (1.75–2.60) | < 0.001 |
| Graft type | |||
| Bone marrow | 1046 | 1.00a | Poverall = 0.002 |
| Peripheral blood | 2534 | 1.33 (1.11–1.61) | 0.002 |
| GVHD prophylaxis | |||
| CNI based with Methotrexate | 2545 | 1.00a | Poverall = 0.03 |
| CNI based with MMF | 586 | 1.34 (1.10–1.64) | 0.004 |
| CNI +- others | 371 | 1.20 (0.95–1.51) | 0.12 |
| Other GVHD prophylaxis | 78 | 1.13 (0.68–1.87) | 0.63 |
| Planned GM or GCSF (within 12 days from transplant)b | |||
| No | 2088 | 1.00a | Poverall < 0.001 |
| Yes | 1492 | 1.32 (1.15–1.52) | < 0.001 |
| Year of transplant | < 0.001 | ||
| Continuous | 3580 | 0.88 (0.84–0.92) | < 0.001 |
|
| |||
| Contrast | |||
| Main effect MK positive vs. other unfavorable | 1.06 (0.79–1.43) | 0.69 | |
| Main effect MK positive vs. intermediate | 1.18 (0.88–1.59) | 0.28 | |
| Main effect other unfavorable vs. intermediate | 1.11 (0.94–1.31) | 0.22 | |
| Age 21–40 MA vs. 41–60 MA | 0.70 (0.58–0.85) | < 0.001 | |
| Age 21–40 MA vs. 41–60 RIC/NMA | 0.72 (0.54–0.96) | 0.03 | |
| Age 21–40 MA vs. 61–64 RIC/NMA | 0.56 (0.40–0.79) | < 0.001 | |
| Age 21–40 MA vs. > 64 RIC/NMA | 0.56 (0.39–0.80) | 0.002 | |
| Age 21–40 MA vs. others | 0.78 (0.55–1.10) | 0.15 | |
| Age 41–60 MA vs. 41–60 RIC/NMA | 1.03 (0.79–1.34) | 0.83 | |
| Age 41–60 MA vs. 61–64 RIC/NMA | 0.80 (0.59–1.09) | 0.16 | |
| Age 41–60 MA vs. > 64 RIC/NMA | 0.80 (0.57–1.11) | 0.19 | |
| Age 41–60 MA vs. others | 1.11 (0.80–1.53) | 0.54 | |
| Age 41–60 RIC/NMA vs. 61–64 RIC/NMA | 0.78 (0.57–1.06) | 0.11 | |
| Age 41–60 RIC/NMA vs. > 64 RIC/NMA | 0.78 (0.56–1.08) | 0.13 | |
| Age 41–60 RIC/NMA vs. others | 1.08 (0.76–1.52) | 0.68 | |
| Age 61–64 RIC/NMA vs. > 64 RIC/NMA | 1.00 (0.70–1.43) | 0.99 | |
| Age 61–64 RIC/NMA vs. others | 1.38 (0.94–2.03) | 0.10 | |
| Age > 64 RIC/NMA vs. others | 1.38 (0.93–2.07) | 0.11 | |
| Karnofsky score < 90% vs. missing | 1.01 (0.71–1.42) | 0.97 | |
| Conditioning TBI +- others vs. Bu + Cy +- others | 1.02 (0.75–1.39) | 0.89 | |
| Conditioning TBI +- others vs. Bu + Flud +- others | 1.19 (0.89–1.59) | 0.23 | |
| Conditioning TBI +- others vs. Flud + Mel +- others | 0.90 (0.65–1.24) | 0.51 | |
| Conditioning TBI +- others vs. other conditioning | 1.26 (0.84–1.88) | 0.27 | |
| Conditioning Bu + Cy +- others vs. Bu + Flud +- others | 1.17 (0.93–1.47) | 0.19 | |
| Conditioning Bu + Cy +- others vs. Flud + Mel +- others | 0.88 (0.63–1.22) | 0.43 | |
| Conditioning Bu + Cy +- others vs. other conditioning | 1.23 (0.82–1.84) | 0.32 | |
| Conditioning Bu + Flud +- others vs. Flud/Mel +- others | 0.75 (0.56–1.01) | 0.06 | |
| Conditioning Bu + Flud +- others vs. other conditioning | 1.05 (0.72–1.55) | 0.79 | |
| Conditioning Flud + Mel +- others vs. other conditioning | 1.40 (0.93–2.12) | 0.11 | |
| HLA matching 8/8 vs. 7/8 | 0.68 (0.56–0.82) | < 0.001 | |
| GVHD prophylaxis CNI based+MMF vs. CNI +- others | 1.12 (0.86–1.46) | 0.41 | |
| GVHD prophylaxis CNI based with MMF vs. other | 1.19 (0.70–2.01) | 0.53 | |
| GVHD prophylaxis CNI +- others vs. other | 1.06 (0.62–1.82) | 0.83 | |
Reference group
GF within 7d: RR=1.39, p<0.001
Table D.
Multivariate analysis of treatment-related mortality for MDS, by monosomal karyotype
| Relative Risk | P-value | ||
|---|---|---|---|
| Main effect: | |||
| Normal | 237 | 1.00a | Poverall < 0.001 |
| MK positive | 219 | 1.80 (1.27–2.54) | < 0.001 |
| Other unfavorable | 416 | 1.37 (0.99–1.90) | 0.06 |
| Intermediate | 606 | 1.01 (0.73–1.39) | 0.97 |
| Favorable | 97 | 0.95 (0.59–1.52) | 0.83 |
| Other significant covariates: | |||
| Age at transplant by conditioning intensity, years | |||
| 0–20 MA | 132 | 1.00a | Poverall < 0.001 |
| 21–40 MA | 210 | 1.62 (1.01–2.58) | 0.04 |
| 41–60 MA | 512 | 2.30 (1.50–3.54) | < 0.001 |
| 41–60 RIC/NMA | 277 | 2.41 (1.53–3.79) | < 0.001 |
| 61–64 RIC/NMA | 158 | 2.18 (1.33–3.57) | 0.002 |
| > 64 RIC/NMA | 113 | 2.69 (1.56–4.66) | < 0.001 |
| Others | 173 | 2.67 (1.65–4.34) | < 0.001 |
| Karnofsky score | |||
| 90–100% | 1032 | 1.00a | Poverall = 0.003 |
| < 90% | 476 | 1.40 (1.16–1.70) | < 0.001 |
| Missing | 67 | 1.20 (0.78–1.87) | 0.41 |
| HLA matching | |||
| HLA-identical sibling | 662 | 1.00a | Poverall < 0.001 |
| Unrelated 8/8 | 704 | 1.11 (0.90–1.36) | 0.32 |
| Unrelated 7/8 | 209 | 1.74 (1.34–2.26) | < 0.001 |
| Disease status at transplant | |||
| Early | 642 | 1.00a | |
| Advanced | 933 | 1.30 (1.08–1.58) | 0.006 |
| Year of transplant | |||
| Continuous | 1575 | 0.91 (0.86–0.96) | < 0.001 |
|
| |||
| Contrast | |||
| Main effect MK positive vs. other unfavorable | 1.31 (0.98–1.76) | 0.07 | |
| Main effect MK positive vs. intermediate | 1.79 (1.34–2.38) | < 0.001 | |
| Main effect MK positive vs. favorable | 1.89 (1.22–2.94) | 0.005 | |
| Main effect other unfavorable vs. intermediate | 1.36 (1.08–1.71) | 0.009 | |
| Main effect other unfavorable vs. favorable | 1.44 (0.96–2.17) | 0.08 | |
| Main effect intermediate vs. favorable | 1.06 (0.71–1.58) | 0.78 | |
| Age 21–40 MA vs. 41–60 MA | 0.70 (0.52–0.95) | 0.02 | |
| Age 21–40 MA vs. 41–60 RIC/NMA | 0.67 (0.48–0.94) | 0.02 | |
| Age 21–40 MA vs. 61–64 RIC/NMA | 0.74 (0.50–1.09) | 0.13 | |
| Age 21–40 MA vs. > 64 RIC/NMA | 0.60 (0.38–0.95) | 0.03 | |
| Age 21–40 MA vs. others | 0.61 (0.42–0.88) | 0.009 | |
| Age 41–60 MA vs. 41–60 RIC/NMA | 0.96 (0.73–1.25) | 0.74 | |
| Age 41–60 MA vs. 61–64 RIC/NMA | 1.06 (0.77–1.46) | 0.74 | |
| Age 41–60 MA vs. > 64 RIC/NMA | 0.85 (0.58–1.27) | 0.43 | |
| Age 41–60 MA vs. others | 0.86 (0.63–1.17) | 0.34 | |
| Age 41–60 RIC/NMA vs. 61–64 RIC/NMA | 1.10 (0.77–1.57) | 0.58 | |
| Age 41–60 RIC/NMA vs. > 64 RIC/NMA | 0.89 (0.59–1.36) | 0.60 | |
| Age 41–60 RIC/NMA vs. others | 0.90 (0.64–1.27) | 0.55 | |
| Age 61–64 RIC/NMA vs. > 64 RIC/NMA | 0.81 (0.51–1.28) | 0.36 | |
| Age 61–64 RIC/NMA vs. others | 0.82 (0.56–1.20) | 0.30 | |
| Age > 64 RIC/NMA vs. others | 1.01 (0.65–1.57) | 0.97 | |
| Karnofsky score < 90% vs. missing | 1.17 (0.74–1.83) | 0.50 | |
| HLA matching 8/8 vs. 7/8 | 0.64 (0.49–0.83) | < 0.001 | |
Reference group
Table E.
Multivariate analysis of overall survival for AML, by monosomal karyotype
| Relative Risk of Death | P-value | ||
|---|---|---|---|
| Main effect: | |||
| Normal | 643 | 1.00a | Poverall < 0.001 |
| MK positive | 240 | 1.67 (1.38–2.01) | < 0.001 |
| Other unfavorable | 1138 | 1.22 (1.06–1.40) | 0.006 |
| Intermediate | 1579 | 0.90 (0.78–1.05) | 0.17 |
| Other significant covariates: | |||
| Age at transplant by conditioning intensity, years | |||
| 0–20 MA | 474 | 1.00a | Poverall < 0.001 |
| 21–40 MA | 811 | 1.06 (0.89–1.27) | 0.50 |
| 41–60 MA | 1228 | 1.43 (1.21–1.69) | < 0.001 |
| 41–60 RIC/NMA | 461 | 1.85 (1.53–2.23) | < 0.001 |
| 61–64 RIC/NMA | 241 | 2.10 (1.69–2.61) | < 0.001 |
| > 64 RIC/NMA | 189 | 2.16 (1.72–2.72) | < 0.001 |
| Others | 196 | 1.29 (1.01–1.66) | 0.04 |
| Karnofsky score | |||
| 90–100% | 2562 | 1.00a | Poverall < 0.001 |
| < 90% | 906 | 1.30 (1.17–1.44) | < 0.001 |
| Missing | 132 | 1.22 (0.98–1.53) | 0.07 |
| HLA matching | |||
| HLA-identical sibling | 1873 | 1.00a | Poverall < 0.001 |
| Unrelated 8/8 | 1276 | 1.18 (1.06–1.31) | 0.002 |
| Unrelated 7/8 | 451 | 1.48 (1.29–1.70) | < 0.001 |
| Year of transplant | |||
| Continuous | 3600 | 0.95 (0.93–0.98) | 0.002 |
|
| |||
| Contrast | |||
| Main effect MK positive vs. other unfavorable | 1.37 (1.15–1.62) | < 0.001 | |
| Main effect MK positive vs. intermediate | 1.84 (1.55–2.19) | < 0.001 | |
| Main effect other unfavorable vs. intermediate | 1.35 (1.20–1.50) | < 0.001 | |
| Age 21–40 MA vs. 41–60 MA | 0.74 (0.65–0.85) | < 0.001 | |
| Age 21–40 MA vs. 41–60 RIC/NMA | 0.58 (0.49–0.68) | < 0.001 | |
| Age 21–40 MA vs. 61–64 RIC/NMA | 0.51 (0.42–0.61) | < 0.001 | |
| Age 21–40 MA vs. > 64 RIC/NMA | 0.49 (0.40–0.60) | < 0.001 | |
| Age 21–40 MA vs. others | 0.82 (0.65–1.04) | 0.10 | |
| Age 41–60 MA vs. 41–60 RIC/NMA | 0.77 (0.67–0.89) | < 0.001 | |
| Age 41–60 MA vs. 61–64 RIC/NMA | 0.68 (0.57–0.81) | < 0.001 | |
| Age 41–60 MA vs. > 64 RIC/NMA | 0.66 (0.55–0.80) | < 0.001 | |
| Age 41–60 MA vs. others | 1.11 (0.89–1.38) | 0.36 | |
| Age 41–60 RIC/NMA vs. 61–64 RIC/NMA | 0.88 (0.72–1.07) | 0.19 | |
| Age 41–60 RIC/NMA vs. > 64 RIC/NMA | 0.85 (0.69–1.05) | 0.13 | |
| Age 41–60 RIC/NMA vs. others | 1.43 (1.13–1.81) | 0.003 | |
| Age 61–64 RIC/NMA vs. > 64 RIC/NMA | 0.97 (0.77–1.22) | 0.80 | |
| Age 61–64 RIC/NMA vs. others | 1.63 (1.26–2.10) | < 0.001 | |
| Age > 64 RIC/NMA vs. others | 1.67 (1.28–2.19) | < 0.001 | |
| Karnofsky score < 90% vs. missing | 1.06 (0.84–1.34) | 0.62 | |
| HLA matching 8/8 vs. 7/8 | 0.80 (0.69–0.91) | 0.001 | |
Reference group
Table F.
Multivariate analysis of overall survival for MDS, by monosomal karyotype
| Relative Risk of death | P-value | ||
|---|---|---|---|
| Main effect: | |||
| Normal | 241 | 1.00a | Poverall < 0.001 |
| MK positive | 221 | 2.02 (1.59–2.59) | < 0.001 |
| Other unfavorable | 423 | 1.39 (1.10–1.77) | 0.006 |
| Intermediate | 611 | 0.96 (0.76–1.22) | 0.73 |
| Favorable | 98 | 1.00 (0.71–1.41) | 1.00 |
| Other significant covariates: | |||
| Age at transplant by conditioning intensity, years | |||
| 0–20 MA | 132 | 1.00a | Poverall < 0.001 |
| 21–40 MA | 212 | 1.32 (0.93–1.86) | 0.12 |
| 41–60 MA | 519 | 2.08 (1.52–2.83) | < 0.001 |
| 41–60 RIC/NMA | 281 | 2.18 (1.57–3.02) | < 0.001 |
| 61–64 RIC/NMA | 160 | 2.05 (1.44–2.91) | < 0.001 |
| > 64 RIC/NMA | 116 | 2.70 (1.83–3.97) | < 0.001 |
| Others | 174 | 2.13 (1.50–3.04) | < 0.001 |
| Karnofsky score | |||
| 90–100% | 1041 | 1.00a | Poverall < 0.001 |
| < 90% | 484 | 1.40 (1.22–1.62) | < 0.001 |
| Missing | 69 | 1.34 (0.99–1.82) | 0.06 |
| HLA matching | |||
| HLA-identical sibling | 668 | 1.00a | Poverall < 0.001 |
| Unrelated 8/8 | 712 | 1.09 (0.94–1.27) | 0.26 |
| Unrelated 7/8 | 214 | 1.62 (1.34–1.97) | < 0.001 |
| Disease status at transplant | |||
| Early | 652 | 1.00a | |
| Advanced | 942 | 1.45 (1.26–1.67) | < 0.001 |
| Year of transplant | |||
| Continuous | 1594 | 0.94 (0.90–0.98) | 0.004 |
|
| |||
| Contrast | |||
| Main effect MK positive vs. other unfavorable | 1.45 (1.19–1.78) | < 0.001 | |
| Main effect MK positive vs. intermediate | 2.11 (1.73–2.58) | < 0.001 | |
| Main effect MK positive vs. favorable | 2.02 (1.48–2.78) | < 0.001 | |
| Main effect other unfavorable vs. intermediate | 1.45 (1.22–1.72) | < 0.001 | |
| Main effect other unfavorable vs. favorable | 1.39 (1.03–1.88) | 0.03 | |
| Main effect intermediate vs. favorable | 0.96 (0.71–1.29) | 0.78 | |
| Age 21–40 MA vs. 41–60 MA | 0.63 (0.50–0.80) | < 0.001 | |
| Age 21–40 MA vs. 41–60 RIC/NMA | 0.60 (0.47–0.78) | < 0.001 | |
| Age 21–40 MA vs. 61–64 RIC/NMA | 0.64 (0.48–0.86) | 0.003 | |
| Age 21–40 MA vs. > 64 RIC/NMA | 0.49 (0.35–0.68) | < 0.001 | |
| Age 21–40 MA vs. others | 0.62 (0.46–0.82) | 0.001 | |
| Age 41–60 MA vs. 41–60 RIC/NMA | 0.95 (0.79–1.15) | 0.62 | |
| Age 41–60 MA vs. 61–64 RIC/NMA | 1.01 (0.81–1.28) | 0.90 | |
| Age 41–60 MA vs. > 64 RIC/NMA | 0.77 (0.59–1.01) | 0.06 | |
| Age 41–60 MA vs. others | 0.97 (0.77–1.23) | 0.82 | |
| Age 41–60 RIC/NMA vs. 61–64 RIC/NMA | 1.06 (0.83–1.37) | 0.63 | |
| Age 41–60 RIC/NMA vs. > 64 RIC/NMA | 0.81 (0.60–1.08) | 0.15 | |
| Age 41–60 RIC/NMA vs. others | 1.02 (0.79–1.32) | 0.86 | |
| Age 61–64 RIC/NMA vs. > 64 RIC/NMA | 0.76 (0.55–1.04) | 0.09 | |
| Age 61–64 RIC/NMA vs. others | 0.96 (0.72–1.27) | 0.78 | |
| Age > 64 RIC/NMA vs. others | 1.27 (0.92–1.74) | 0.14 | |
| Karnofsky score < 90% vs. missing | 1.05 (0.77–1.43) | 0.77 | |
| HLA matching 8/8 vs. 7/8 | 0.67 (0.55–0.81) | < 0.001 | |
Reference group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: There are no relevant conflicts of interest to disclose.
References
- 1.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 2.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 3.Breems DA, Van Putten WLJ, De Greef GE, et al. Monosomal Karyotype in Acute Myeloid Leukemia: A Better Indicator of Poor Prognosis Than a Complex Karyotype. J Clin Oncol. 2008;26:4791–4797. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 5.Schanz J, Tuchler H, Sole F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30:820–829. doi: 10.1200/JCO.2011.35.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schanz J, Tuchler H, Sole F, et al. Monosomal karyotype in MDS: explaining the poor prognosis? Leukemia. 2013;27:1988–1995. doi: 10.1038/leu.2013.187. [DOI] [PubMed] [Google Scholar]
- 7.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 8.Tallman MS, Dewald GW, Gandham S, et al. Impact of cytogenetics on outcome of matched unrelated donor hematopoietic stem cell transplantation for acute myeloid leukemia in first or second complete remission. Blood. 2007;110:409–417. doi: 10.1182/blood-2006-10-043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquini MC, Wang Z, Horowitz M, Gale RP. Report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current Uses and Outcomes of Hematopoietic Cell Transplant for Blood and Bone Marrow Disorders. In: Cecka JM, Terazaki PI, editors. Clinical Transplants 2010. Los Angeles: The Terasaki Foundation Laboratory; 2010. 2011. [PubMed] [Google Scholar]
- 10.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 11.Chevallier P, Labopin M, Milpied N, et al. Impact of cytogenetics risk on outcome after reduced intensity conditioning allo-SCT from an HLA-identical sibling for patients with AML in first CR: a report from the acute leukemia working party of EBMT. Bone Marrow Transplant. 2012;47:1442–1447. doi: 10.1038/bmt.2012.55. [DOI] [PubMed] [Google Scholar]
- 12.Lazarevic V, Horstedt AS, Johansson B, et al. Incidence and prognostic significance of karyotypic subgroups in older patients with acute myeloid leukemia: the Swedish population-based experience. Blood Cancer J. 2014;4:e188. doi: 10.1038/bcj.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medeiros BC, Othus M, Fang M, Roulston D, Appelbaum FR. Prognostic impact of monosomal karyotype in young adult and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG) experience. Blood. 2010;116:2224–2228. doi: 10.1182/blood-2010-02-270330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oran B, Dolan M, Cao Q, Brunstein C, Warlick E, Weisdorf D. Monosomal karyotype provides better prognostic prediction after allogeneic stem cell transplantation in patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2011;17:356–364. doi: 10.1016/j.bbmt.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Choi Y, Lee J-H, Seo E-J, et al. Monosomal karyotype in acute myeloid leukemia and the role of allogeneic hematopoietic cell transplantation. Annals of Hematology. 2015:1–7. doi: 10.1007/s00277-014-2286-7. [DOI] [PubMed] [Google Scholar]
- 16.Cornelissen JJ, Breems D, van Putten WL, et al. Comparative analysis of the value of allogeneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J Clin Oncol. 2012;30:2140–2146. doi: 10.1200/JCO.2011.39.6499. [DOI] [PubMed] [Google Scholar]
- 17.Fang M, Storer B, Estey E, et al. Outcome of patients with acute myeloid leukemia with monosomal karyotype who undergo hematopoietic cell transplantation. Blood. 2011;118:1490–1494. doi: 10.1182/blood-2011-02-339721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Gelder M, de Wreede LC, Schetelig J, et al. Monosomal karyotype predicts poor survival after allogeneic stem cell transplantation in chromosome 7 abnormal myelodysplastic syndrome and secondary acute myeloid leukemia. Leukemia. 2012;27:879–888. doi: 10.1038/leu.2012.297. [DOI] [PubMed] [Google Scholar]
- 19.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayser S, Zucknick M, Döhner K, et al. Monosomal karyotype in adult acute myeloid leukemia: prognostic impact and outcome after different treatment strategies. Blood. 2012;119:551–558. doi: 10.1182/blood-2011-07-367508. [DOI] [PubMed] [Google Scholar]
- 21.Haferlach C, Alpermann T, Schnittger S, et al. Prognostic value of monosomal karyotype in comparison to complex aberrant karyotype in acute myeloid leukemia: a study on 824 cases with aberrant karyotype. Blood. 2012;119:2122–2125. doi: 10.1182/blood-2011-10-385781. [DOI] [PubMed] [Google Scholar]
- 22.Grossmann V, Schnittger S, Kohlmann A, et al. A novel hierarchical prognostic model of AML solely based on molecular mutations. Blood. 2012;120:2963–2972. doi: 10.1182/blood-2012-03-419622. [DOI] [PubMed] [Google Scholar]
- 23.Rucker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119:2114–2121. doi: 10.1182/blood-2011-08-375758. [DOI] [PubMed] [Google Scholar]
- 24.Breems DA, Van Putten WL, Lowenberg B. The impact of abn(17p) and monosomy −5/del(5q) on the prognostic value of the monosomal karyotype in acute myeloid leukemia. Blood. 2013;121:3056–3057. doi: 10.1182/blood-2013-01-475012. [DOI] [PubMed] [Google Scholar]
- 25.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 26.Nazha A, Kantarjian HM, Bhatt VR, et al. Prognostic implications of chromosome 17 abnormalities in the context of monosomal karyotype in patients with acute myeloid leukemia and complex cytogenetics. Clin Lymphoma Myeloma Leuk. 2014;14:163–171. doi: 10.1016/j.clml.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaillard JB, Chiesa J, Reboul D, et al. Monosomal karyotype routinely defines a poor prognosis subgroup in acute myeloid leukemia and is frequently associated with TP53 deletion. Leuk Lymphoma. 2011;53:336–337. doi: 10.3109/10428194.2011.608453. [DOI] [PubMed] [Google Scholar]
- 28.Chen MH, Atenafu E, Craddock KJ, Brandwein J, Chang H. CD11b expression correlates with monosomal karyotype and predicts an extremely poor prognosis in cytogenetically unfavorable acute myeloid leukemia. Leuk Res. 2012;37:122–128. doi: 10.1016/j.leukres.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Ahn HK, Jang JH, Kim K, et al. Monosomal karyotype in acute myeloid leukemia predicts adverse treatment outcome and associates with high functional multidrug resistance activity. Am J Hematol. 2011;87:37–41. doi: 10.1002/ajh.22193. [DOI] [PubMed] [Google Scholar]
- 30.Boudry-Labis E, Roche-Lestienne C, Nibourel O, et al. Neurofibromatosis-1 gene deletions and mutations in de novo adult acute myeloid leukemia. Am J Hematol. 2013;88:306–311. doi: 10.1002/ajh.23403. [DOI] [PubMed] [Google Scholar]
- 31.Perrot A, Luquet I, Pigneux A, et al. Dismal prognostic value of monosomal karyotype in elderly patients with acute myeloid leukemia: a GOELAMS study of 186 patients with unfavorable cytogenetic abnormalities. Blood. 2011;118:679–685. doi: 10.1182/blood-2010-09-307264. [DOI] [PubMed] [Google Scholar]
- 32.Yang XF, Sun AN, Yin J, et al. Monosomal karyotypes among 1147 Chinese patients with acute myeloid leukemia: prevalence, features and prognostic impact. Asian Pac J Cancer Prev. 2013;13:5421–5426. doi: 10.7314/apjcp.2012.13.11.5421. [DOI] [PubMed] [Google Scholar]
- 33.Cornelissen JJ, Breems D, van Putten WL, et al. Comparative analysis of the value of allogeneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J Clin Oncol. 2012;30:2140–2146. doi: 10.1200/JCO.2011.39.6499. [DOI] [PubMed] [Google Scholar]
- 34.Armand P, Kim HT, Zhang MJ, et al. Classifying cytogenetics in patients with acute myelogenous leukemia in complete remission undergoing allogeneic transplantation: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2012;18:280–288. doi: 10.1016/j.bbmt.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemmati PG, Schulze-Luckow A, Terwey TH, et al. Cytogenetic risk grouping by the monosomal karyotype classification is superior in predicting the outcome of acute myeloid leukemia undergoing allogeneic stem cell transplantation in complete remission. Eur J Haematol. 2013;92:102–110. doi: 10.1111/ejh.12216. [DOI] [PubMed] [Google Scholar]
- 36.Middeke JM, Beelen D, Stadler M, et al. Outcome of high-risk acute myeloid leukemia after allogeneic hematopoietic cell transplantation: negative impact of abnl(17p) and −5/5q. Blood. 2012;120:2521–2528. doi: 10.1182/blood-2012-03-417972. [DOI] [PubMed] [Google Scholar]
- 37.van Gelder M, de Wreede LC, Schetelig J, et al. Monosomal karyotype predicts poor survival after allogeneic stem cell transplantation in chromosome 7 abnormal myelodysplastic syndrome and secondary acute myeloid leukemia. Leukemia. 2013;27:879–888. doi: 10.1038/leu.2012.297. [DOI] [PubMed] [Google Scholar]
- 38.Sloand EM, Yong AS, Ramkissoon S, et al. Granulocyte colony-stimulating factor preferentially stimulates proliferation of monosomy 7 cells bearing the isoform IV receptor. Proc Natl Acad Sci U S A. 2006;103:14483–14488. doi: 10.1073/pnas.0605245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing R, Li C, Gale RP, et al. Monosomal karyotype is an independent predictor of survival in patients with higher-risk myelodysplastic syndrome. American Journal of Hematology. 2014;89:E163–E168. doi: 10.1002/ajh.23801. [DOI] [PubMed] [Google Scholar]
- 40.Deeg HJ, Scott BL, Fang M, et al. Five-group cytogenetic risk classification, monosomal karyotype, and outcome after hematopoietic cell transplantation for MDS or acute leukemia evolving from MDS. Blood. 2012;120:1398–1408. doi: 10.1182/blood-2012-04-423046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koenecke C, Gohring G, de Wreede LC, et al. Impact of the revised International Prognostic Scoring System, cytogenetics and monosomal karyotype on outcome after allogeneic stem cell transplantation for myelodysplastic syndromes and secondary acute myeloid leukemia evolving from myelodysplastic syndromes: a retrospective multicenter study of the European Society of Blood and Marrow Transplantation. Haematologica. 2014;3 doi: 10.3324/haematol.2014.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cluzeau T, Mounier N, Karsenti JM, et al. Monosomal karyotype improves IPSS-R stratification in MDS and AML patients treated with Azacitidine. Am J Hematol. 2013;88:780–783. doi: 10.1002/ajh.23509. [DOI] [PubMed] [Google Scholar]
- 43.Della Porta MG, Alessandrino EP, Bacigalupo A, et al. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood. 2014;123:2333–2342. doi: 10.1182/blood-2013-12-542720. [DOI] [PubMed] [Google Scholar]
- 44.Ustun C, Trottier BJ, Sachs Z, et al. Monosomal Karyotype at the Time of Diagnosis or Transplantation Predicts Outcomes of Allogeneic Hematopoietic Cell Transplantation in Myelodysplastic Syndrome. Biology of Blood and Marrow Transplantation. 2015 doi: 10.1016/j.bbmt.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pagel JM, Gooley TA, Rajendran J, et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114:5444–5453. doi: 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


