Abstract
AIM: To observe the effect of β-ionone on the proliferation of human gastric adenocarcinoma cell line SGC-7901 and the inhibition of metalloproteinase.
METHODS: Using growth inhibition, Zymograms assays and reverse transcription-polymerase-chain reaction (RT-PCR), we examined cell growth rates, activities of matrix metalloproteinases-2 (MMP-2) and -9 (MMP-9), and expression of metalloproteinases-1 (TIMP-1) and -2 (TIMP-2) in SGC-7901 cells after the treatment with β-ionone for 24 h and 48 h, respectively.
RESULTS: β-ionone had an inhibitory effect on the growth of SGC-7901 cells. Eight days after the treatment with β-ionone at concentrations of 25, 50, 100 and 200 μmol/L, the inhibition rates were 25.9%, 28.2%, 74.4% and 90.1%, respectively. The IC50 value of β-ionone for SGC-7901 cells was estimated to be 89 μmol/L. The effects of β-ionone on MMP-2 and MMP-9 activities in SGC-7901 cells were not observed. However, the levels of TIMP-1 and TIMP-2 transcripts were elevated in cells treated with β-ionone in a dose-dependent manner.
CONCLUSION: β-ionone can inhibit the proliferation of SGC-7901 cells, upregulate the expression of TIMP-1 and TIMP-2 expression, and may influence metastasis of cancer.
INTRODUCTION
Epidemiological data showed that regular consumption of fruits and vegetables was associated with a reduced risk of chronic diseases such as cancer and cardiovascular diseases[1-4]. Isoprenoid is an important group of nutritious elements found in fruits,vegetables and cereal grains, giving rise to about 22 000 secondary products during its metabolism in these plants. It has been found that these compounds shared a common precursor, mevalonic acid[14]. Isoprenoids have been shown to suppress chemically-induced carcinogenesis[5-10] and the growth of cancer cells[11-13]. β-ionone, an end-ring analog of β-carotenoid, represents a subclass of cyclic isoprenoids and has been demonstrated to have an anticancer effect[15]. β-ionone was effective in chemoprevention of 7,12-dimethylben[a] anthracene-induced mammary carcinogenesis in SD rats, with its tumor multiplicity reduced by 45%[16]. It has also been found to be associated with growth inhibition of melanoma and breast cancer cells as well as metastasis of tumor cells[17-20]. However, the exact mechanisms remain to be clarified, and the effect of β-ionone on gastric cancer cells is unknown.
Chemoprevention has been an approach to treat advanced cancers[21-25]. Gastric cancer is one of the most common malignancies in China and in other parts of the world[26-34]. Understanding the mechanisms of the possible inhibitory effect of β-ionone on proliferation of gastric adenocarcinoma cells could provide a way to prevent this disease. Therefore, in this study, we studied the effect of β-ionone on the proliferation of human gastric adenocarcinoma cell line SGC-7901 and on the regulative factors of tissue metalloproteinases and investigated the underlying mechanism.
MATERIALS AND METHODS
Cell culture
Human gastric adenocarcinoma cell line SGC-7901, provided by Beijing Cancer Research Institute, was grown in RPMI 1640 medium (Gibco BRL, Life Technologies Inc, Gaithersburg, MD, USA), supplemented with 100 ml/L calf serum, 100×103 U/L penicillin and 100 mg/L streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
IC50 and cell growth assessment
β-ionone (purity > 95%) (Sigma, USA) was dissolved in absolute ethanol and further diluted to the concentrations of 25, 50, 100 and 200 μmol/L, respectively.
The SGC-7901 cells were seeded into six 24-well plates (Nuc, Denmark) with 2×104 cells/well after 24 h culture. Twenty-four hour later, the medium was replaced with the media supplemented with at different concentrations of β-ionone. In the next 8 days, the cells were treated with a trysin-EDTA solution at 37 °C for 2 minutes and harvested from 3 wells per day for each plate. The cells were pelleted by a short spin at 1 000 g and resuspended in phosphate-buffered saline (PBS). Typan blue exclusion test was used to count viable cells by a hemocytometer. The number of cells at 24 h was deducted from the final cell counts to provide an estimate of the net increase. The β-ionone concentration required to inhibit the net increase in the 48 h cell count by 50% (IC50) was measured by plotting data obtained from three or more times. The means were obtained on each of the eight days and used to draw a cell growth curve. The inhibitory rate (IR) was calculated according to the formula: IR (%) = (total number of cells in negative control-number of cells in test group)/total number of cells in negative control×100%.
Zymograms assay for activities of gelatinase
Although many kinds of proteinase are involved in tumor metastasis, members of the matrix metalloproteinase (MMPs, gelatinase) and TIMP families, play a pivotal role in these events. MMPs are a group of zinc-dependent endopeptidase molecules that have the potential to degrade proteins of the extracellular matrix. MMP activity was under regulation at several levels, including activation of the MMP zymogens and specific inhibition by the tissue inhibitors of metalloproteinase (TIMPs)[54]. We determined the activities of gelatinase in SGC-7901 cells treated with β-ionone.
The SGC-7901 cells were seeded in 100 ml bottles, each containing 5×106 cells. After incubation for 24 h, the medium was replaced with 400 μL of serum-free medium supplemented with β-ionone at different concentrations for 24 h and 48 h. The serum-free media were loaded onto a 100 g/L SDS-polyacrylamide gel co-polymerized with 1 g/L gelatin (Amresco Corp, USA) and separated under a non-denaturing condition for 3-4 h. Then, the gels were incubated in 25 g/L Triton X-100 (Sigma, USA) for 1 h and subsequently in a substrate buffer (50 μmol/L Tris, PH7.5, containing 10 mmol/L CaCl2, 200 mmol/L NaCl and 1 μmol/L ZnCl2) for 12 to 16 h at 37 °C. Finally, the gels were stained in a solution containing 1 g/L Commassie blue R250, 450 g/L methanol and 100 g/L acetic acid, and the gelatinolytic activity was indicated by bands in a blue background.
RT-PCR
Tissue inhibitor of TIMP-1 and TIMP-2 were related to several tumorigenic processes in lung, stomach, and mammary gland[56]. We examined the expression of TIMP-1 and TIMP-2 in SGC-7901 cells exposed to different concentrations of β-ionone.
SGC-7901 cells (5×106) were incubated at different concentrations of β-ionone for 24 h and 48 h, the total cellular RNA was isolated. Concentrations and purity of total RNA were determined. RT-PCR was performed following the manufacturer’s instructions (Takara Biotech, Dalian, China). Total RNA (5 µg) and AMV reverse transcriptase XL were used to synthesize cDNA. Twenty-five μL PCR mixture containing 4 μL of RT reaction product, 2.5 U Taq DNA polymerase and 20 pmol primers, was heated for 5 min at 94 °C for pre-denaturation, and then subjected to 35 PCR cycles, denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s for and extension at 72 °C for 45 s, in a PTC-100 thermocycler (MJ Research, USA). TIMP-1 and TIMP-2 genes were amplified with specific primers, with the gene for β-actin as an internal control (Table 1). The amplified products were resolved in a 20 g/L agarose gel containing ethidium bromide, and visualized under ultraviolet light. The density and area of each band were analyzed using the ChemiImagerTM 4 000 digital system (Alpha Innotech Corp, USA).
Table 1.
Primer sequences and size of TIMP-1 and TIMP-2 in expected PCR products for RT-PCR
| Genes | Primer sequences | Product size (bp) |
| TIMP-1 | Sense: 5’-CTGTTGGCTGTGAGGAATGCACAG-3’ | 106 |
| Antisense: 5’-TTCAGAGCCTTGGAGGAGCTGGTC-3’ | ||
| TIMP-2 | Sense: 5’-AGACGTAGTGATCGGGCCA-3’ | 490 |
| Antisense: 5’-GTACCACGCGCAAGAACCT-3’ | ||
| β-actin | Sense: 5’-AAGGATTCCTATGTGGGC-3’ | 532 |
| Antisense: 5’-CATCTCTTGCTCGAAGTC-3’ |
Statistical analysis
Student’t test was used for analysis of data. P < 0.05 was considered statistically significant.
RESULTS
Cell growth inhibition of β-ionone
The inhibitory effect of β-ionone on the growth of SGC-7901 cells was shown in Figure 1. Growth of the cells in the media containing 200 μmol/L and 100 μmol/L β-ionone was markedly slower than that of the negative control within 8 d (P < 0.01). The change was less obvious the cells treated with 50 μmol/L and 25 μmol/L of β-ionone. The inhibitory rates were 25.9%, 28.2%, 74.4% and 90.1%, respectively. The IC50 of β-ionone was 89 μmol/L.
Figure 1.
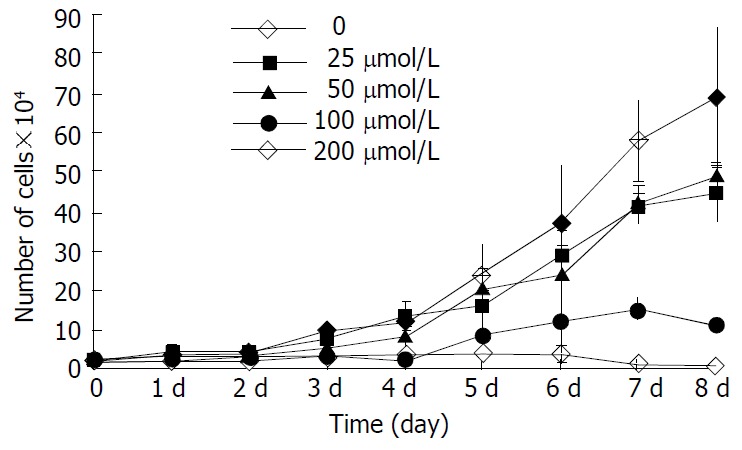
Proliferation kinetics of SGC-7901 cells treated at vari-ous concentrations of β-ionone.
Expression of type IV collagenases
The effects of β-ionone on type IV collagenase activities (97kD for MMP-9 and 72kD for MMP-2) in SGC-7901 cells were presented in Figure 2. In the serum-free supernatants of SGC-7901 cells preincubated in the media supplemented with various concentrations of β-ionone, the activities of type IV collagenases (both MMP-9 and MMP-2) were not different in comparison with the negative control. No effect of β-ionone on the activities of type IV collagenases in SGC-7901 cells was shown.
Figure 2.

Effect of β-ionone on gelatinase/type IV collagenase secretion (97kD for MMP-9 and 72kD for MMP-2) in SGC-7901 cells.
Levels of TIMP-1 and TIMP-2 mRNA in SGC-7901 cells
As shown in Figures 3 and 4, the levels of TIMP-1 and TIMP-2 transcripts in SGC-7901 cells were gradually elevated with increase of the β-ionone concentration, indicating a upregulation effect of β-ionone on the expression of TIMP-1 and TIMP-2 in SGC-7901 cells.
Figure 3.
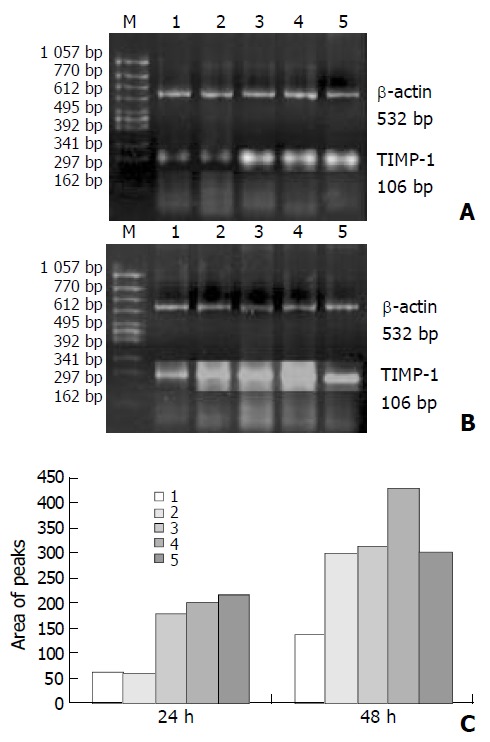
RT-PCR result: expression of TIMP-1 mRNA in SGC-7901 cells treated by β-ionone for 24 h (A) and 48 h (B). C: area of peaks; Lanes 1-5: 0, 25, 50, 100 and 200 μmol/L of β-ionone; M: molecular weight markers.
Figure 4.
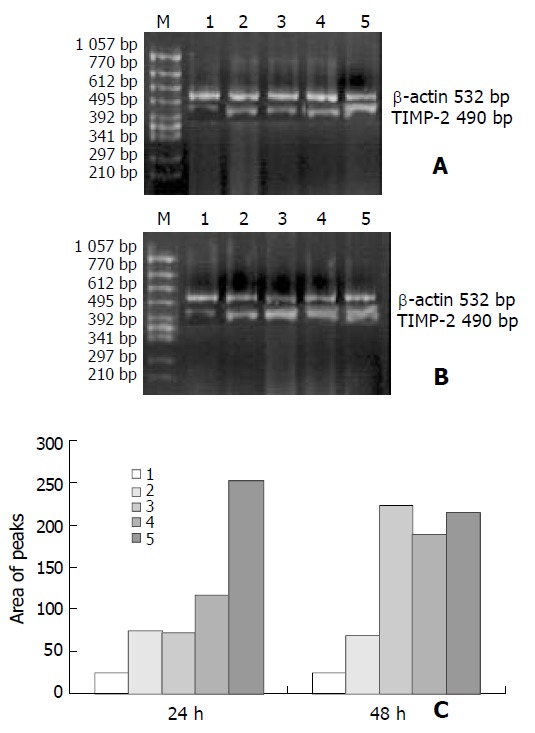
RT-PCR results showing expression of TIMP-2 mRNA in SGC-7901 cells treated by β-ionone for 24 h (A) and 48 h (B). C: chart of area of peaks; Lanes 1-5: 0, 25, 50, 100 and 200 μmol/L of β-ionone; M: molecular weight markers.
DISCUSSION
Interactions between cells and extracellular matrix (ECM) were critical for many cellular functions including division, migration, differentiation, and apoptosis[35]. In different mature normal tissues, ECM is specified by its structures and compositions to maintain tissue homeostasis and cellular quiescence. It have been found that remodeling of the ECM occurred during embryonic development and certain normal pathological processes such as wound-healing[36,37]. However, restructuring of the ECM has also been implicated in the pathogenesis of various human diseases including impaired wound-healing and fibrosis[38], rheumatoid arthritis[39], restenosis following balloon angioplasty[40], atherosclerosis[41], tumor development, invasion and metastasis[35,42].
Tumor invasion is a complex biological process, during which tumor cells detach from the primary tumor and infiltrate the surrounding tissues. This process has been found to require loss of cell contacts between tumor cells, active cell migration, adhesion to ECM and proteolytic degradation of the ECM[43,44]. Different molecules including cadherins, integrins, proteases, and growth factors were implicated in the regulation of invasion of tumor cells[44].
A major group of enzymes responsible for ECM degradation in cancer tissue has been found to be the MMP family, including collagenases, gelatinase, and stromelysins[45-49]. Collagenases can cleave fibrillar collagens at neutral conditions and play an important role in matrix remodeling. The largest sub-family of the MMPs is membrane-type (MT). MT-MMPs play a dual role in cell surface proteolysis. They can cleave a variety of extracellular components, and activate some secreted MMPs. Evidence has suggested that proteolytic activities at cell surface could promote cell invasion[50]. MMP-2, a 72-kDa type IV collagenase, is a cell surface-associated type I collagen-degrading MMP, which was found to designate gelatinase A[51]. Its overexpression has been observed in different types of tumors, and it was believed to be involved in tumor metastasis, primary tumor growth and angiogenesis[52]. MMP-2 is also regarded as a type IV collagenase involved in cell invasion across basement membrane. Thus, serum and tissue MMP profiles have been used as prognostic factors in certain types of malignant tumors including gastric and breast cancers[53]. MMP-9, a 97-kDa type IV collagenase also designating gelatinase B, was found to be associated with tumor invasion as well, its expression has been proven to be correlated with the differentiation grade of some malignancies[54]. In the present study, we did not find any effect of β-ionone on MMP-9 and MMP-2 activities in SGC-7901 cells. The growth inhibition on SGC-7901 cells appeared to be independence of the type IV collagenase activity.
TIMPs (TIMP-1, -2, -3 and -4) have been found to be the key regulators of MMP activity and ECM degradation[60]. The MMP inhibitors, TIMP-1 and TIMP-2 have been associated to several tumorigenic processes including development, invasion and metastasis of bronchial cancer[55-59]. Recent transgenic animal studies have demonstrated that alteration of the MMP/TIMP balance in vivo in favor of TIMP-1 activity could block neoplastic proliferation in the SV40 T antigen-induced murine hepatocellular carcinoma[61]. Active MT1-MMP was found to bind to amino-terminal domain of TIMP-2[62], whereas an interaction between the carboxyl-terminal domains of TIMP-2 and pro-MMP-2 was also described[63]. TIMP-2 might prevent SH2-protein-tyrosine phosphatase-1 (SHP-1) dissociation from immunoprecipitable epidermal growth factor receptor complex and a selective increase in total SHP-1 activity[64]. Our results showed that β-ionone might increase the expression of TIMP-1 and TIMP-2 in SGC-7901 cells. Clearly, further studies are needed to elucidate whether β-ionone inhibits metastasis of the cancer cells.
In summary, β-ionone can inhibit SGC-7901 cell growth and proliferation and has an effect on upregulating TIMP-1 and TIMP-2 expression in SGC-7901 cells. It seems to be related to potential tumor metastasis in SGC-7901 cells induced by β-ionone. However, the mechanism of the inhibitory effect of β-ionone inhibits cell proliferation remains to be clarified.
ACKNOWLEDGEMENT
We thank Dr. Dong-Yan Jin for his critically reading and revising of the manuscript.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30200229 and the Youth Foundation of Harbin Medical University, China
Edited by Ren SY and Wang XL
References
- 1.Michaud DS, Pietinen P, Taylor PR, Virtanen M, Virtamo J, Albanes D. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br J Cancer. 2002;87:960–965. doi: 10.1038/sj.bjc.6600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito LS, Inoue M, Tajima K, Yamamura Y, Kodera Y, Hirose K, Takezaki T, Hamajima N, Kuroishi T, Tominaga S. Dietary factors and the risk of gastric cancer among Japanese women: a comparison between the differentiated and non-differentiated subtypes. Ann Epidemiol. 2003;13:24–31. doi: 10.1016/s1047-2797(02)00269-7. [DOI] [PubMed] [Google Scholar]
- 3.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296:695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 4.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 5.Elson CE. Suppression of mevalonate pathway activities by dietary isoprenoids: protective roles in cancer and cardiovascular disease. J Nutr. 1995;125:1666S–1672S. doi: 10.1093/jn/125.suppl_6.1666S. [DOI] [PubMed] [Google Scholar]
- 6.Elson CE, Yu SG. The chemoprevention of cancer by mevalonate-derived constituents of fruits and vegetables. J Nutr. 1994;124:607–614. doi: 10.1093/jn/124.5.607. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Huang Y, Li J, Hu W, Aziz R, Tang MS, Sun N, Cassady J, Stoner GD. Inhibition of benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor kappaB by black raspberry extracts. Cancer Res. 2002;62:6857–6863. [PubMed] [Google Scholar]
- 8.Serafini M, Bellocco R, Wolk A, Ekström AM. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology. 2002;123:985–991. doi: 10.1053/gast.2002.35957. [DOI] [PubMed] [Google Scholar]
- 9.Pandey M, Shukla VK. Diet and gallbladder cancer: a case-control study. Eur J Cancer Prev. 2002;11:365–368. doi: 10.1097/00008469-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Devasena T, Menon VP. Enhancement of circulatory antioxidants by fenugreek during 1,2-dimethylhydrazine-induced rat colon carcinogenesis. J Biochem Mol Biol Biophys. 2002;6:289–292. doi: 10.1080/10258140290030915. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2002;23:1869–1876. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- 12.Ohyama K, Akaike T, Hirobe C, Yamakawa T. Cytotoxicity and apoptotic inducibility of Vitex agnus-castus fruit extract in cultured human normal and cancer cells and effect on growth. Biol Pharm Bull. 2003;26:10–18. doi: 10.1248/bpb.26.10. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, Packer L. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr. 2003;77:160–166. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 14.Bach TJ. Some new aspects of isoprenoid biosynthesis in plants--a review. Lipids. 1995;30:191–202. doi: 10.1007/BF02537822. [DOI] [PubMed] [Google Scholar]
- 15.Mo H, Elson CE. Apoptosis and cell-cycle arrest in human and murine tumor cells are initiated by isoprenoids. J Nutr. 1999;129:804–813. doi: 10.1093/jn/129.4.804. [DOI] [PubMed] [Google Scholar]
- 16.Yu SG, Anderson PJ, Elson CE. Efficacy of β-ionone in the chemoprevention of rat mammary carcinogenesis. J Agric Food Chem. 1995;43:2144–2147. [Google Scholar]
- 17.He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr. 1997;127:668–674. doi: 10.1093/jn/127.5.668. [DOI] [PubMed] [Google Scholar]
- 18.Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, Taniguchi M, Baba K. Antitumor and antimetastatic effects on liver of triterpenoid fractions of Ganoderma lucidum: mechanism of action and isolation of an active substance. Anticancer Res. 2002;22:3309–3318. [PubMed] [Google Scholar]
- 20.Dabrosin C, Chen J, Wang L, Thompson LU. Flaxseed inhibits metastasis and decreases extracellular vascular endothelial growth factor in human breast cancer xenografts. Cancer Lett. 2002;185:31–37. doi: 10.1016/s0304-3835(02)00239-2. [DOI] [PubMed] [Google Scholar]
- 21.Tovey FI, Hobsley M. Post-gastrectomy patients need to be followed up for 20-30 years. World J Gastroenterol. 2000;6:45–48. doi: 10.3748/wjg.v6.i1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G, et al. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248–253. doi: 10.3748/wjg.v7.i2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu WX, Qin XY, Liu H, Wang CP. Clinicopathological analysis of patients with gastric cancer in 1200 cases. World J Gastroenterol. 2001;7:281–284. doi: 10.3748/wjg.v7.i2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng DJ. progress of gastric cancer etiology: N-nitrosamides 1999s. World J Gastroenterol. 2000;6:613–618. doi: 10.3748/wjg.v6.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Liu FK, Li X, Li JS, Xu GX. Inhibitory effect of endostatin expressed by human liver carcinoma SMMC7721 on endothelial cell proliferation in vitro. World J Gastroenterol. 2002;8:253–257. doi: 10.3748/wjg.v8.i2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao WX, Ou JM, Fei XF, Zhu ZG, Yin HR, Yan M, Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol. 2002;8:230–232. doi: 10.3748/wjg.v8.i2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JR, Chen BQ, Yang YM, Wang XL, Xue YB, Zheng YM, Liu RH. Effect of apoptosis on gastric adenocarcinoma cell line SGC-7901 induced by cis-9, trans-11-conjugated linoleic acid. World J Gastroenterol. 2002;8:999–1004. doi: 10.3748/wjg.v8.i6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Lu YY. Applying a highly specific and reproducible cDNA RDA method to clone garlic up-regulated genes in human gastric cancer cells. World J Gastroenterol. 2002;8:213–216. doi: 10.3748/wjg.v8.i2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun ZJ, Pan CE, Liu HS, Wang GJ. Anti-hepatoma activity of resveratrol in vitro. World J Gastroenterol. 2002;8:79–81. doi: 10.3748/wjg.v8.i1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901) World J Gastroenterol. 2002;8:224–229. doi: 10.3748/wjg.v8.i2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR, et al. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54–59. doi: 10.3748/wjg.v8.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao F, Yi J, Shi GY, Li H, Shi XG, Tang XM. The sensitivity of digestive tract tumor cells to As2O3 is associated with the inherent cellular level of reactive oxygen species. World J Gastroenterol. 2002;8:36–39. doi: 10.3748/wjg.v8.i1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen BQ, Xue YB, Liu JR, Yang YM, Zheng YM, Wang XL, Liu RH. Inhibition of conjugated linoleic acid on mouse forestomach neoplasia induced by benzo (a) pyrene and chemopreventive mechanisms. World J Gastroenterol. 2003;9:44–49. doi: 10.3748/wjg.v9.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu K, Li Y, Zhao Y, Shan YJ, Xia W, Yu WP, Zhao L. Roles of Fas signaling pathway in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:982–986. doi: 10.3748/wjg.v8.i6.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 36.Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann N Y Acad Sci. 1998;857:110–118. doi: 10.1111/j.1749-6632.1998.tb10111.x. [DOI] [PubMed] [Google Scholar]
- 37.Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994;8:823–831. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 38.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 39.Cawston T. Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol Med Today. 1998;4:130–137. doi: 10.1016/s1357-4310(97)01192-1. [DOI] [PubMed] [Google Scholar]
- 40.Batchelor WB, Robinson R, Strauss BH. The extracellular matrix in balloon arterial injury: a novel target for restenosis prevention. Prog Cardiovasc Dis. 1998;41:35–49. doi: 10.1016/s0033-0620(98)80021-2. [DOI] [PubMed] [Google Scholar]
- 41.Newby AC, Zaltsman AB. Fibrous cap formation or destruction--the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res. 1999;41:345–360. [PubMed] [Google Scholar]
- 42.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mareel MM, Van Roy FM, Bracke ME. How and when do tumor cells metastasize. Crit Rev Oncog. 1993;4:559–594. [PubMed] [Google Scholar]
- 44.Engers R, Gabbert HE. Mechanisms of tumor metastasis: cell biological aspects and clinical implications. J Cancer Res Clin Oncol. 2000;126:682–692. doi: 10.1007/s004320000148. [DOI] [PubMed] [Google Scholar]
- 45.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 46.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 47.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 49.Onodera S, Nishihira J, Iwabuchi K, Koyama Y, Yoshida K, Tanaka S, Minami A. Macrophage migration inhibitory factor up-regulates matrix metalloproteinase-9 and -13 in rat osteoblasts. Relevance to intracellular signaling pathways. J Biol Chem. 2002;277:7865–7874. doi: 10.1074/jbc.M106020200. [DOI] [PubMed] [Google Scholar]
- 50.Murphy G, Gavrilovic J. Proteolysis and cell migration: creating a path. Curr Opin Cell Biol. 1999;11:614–621. doi: 10.1016/s0955-0674(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 51.Zhuge Y, Xu J. Rac1 mediates type I collagen-dependent MMP-2 activation. role in cell invasion across collagen barrier. J Biol Chem. 2001;276:16248–16256. doi: 10.1074/jbc.m010190200. [DOI] [PubMed] [Google Scholar]
- 52.Sato H, Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem. 1996;119:209–215. doi: 10.1093/oxfordjournals.jbchem.a021223. [DOI] [PubMed] [Google Scholar]
- 53.McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. 2000;6:149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- 54.Rao JS, Yamamoto M, Mohaman S, Gokaslan ZL, Fuller GN, Stetler-Stevenson WG, Rao VH, Liotta LA, Nicolson GL, Sawaya RE. Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp Metastasis. 1996;14:12–18. doi: 10.1007/BF00157681. [DOI] [PubMed] [Google Scholar]
- 55.Brand K. Cancer gene therapy with tissue inhibitors of metalloproteinases (TIMPs) Curr Gene Ther. 2002;2:255–271. doi: 10.2174/1566523024605564. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–2252. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- 57.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giannelli G, Antonaci S. Gelatinases and their inhibitors in tumor metastasis: from biological research to medical applications. Histol Histopathol. 2002;17:339–345. doi: 10.14670/HH-17.339. [DOI] [PubMed] [Google Scholar]
- 59.Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003;36:128–137. doi: 10.5483/bmbrep.2003.36.1.128. [DOI] [PubMed] [Google Scholar]
- 60.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 61.Martin DC, Sanchez-Sweatman OH, Ho AT, Inderdeo DS, Tsao MS, Khokha R. Transgenic TIMP-1 inhibits simian virus 40 T antigen-induced hepatocarcinogenesis by impairment of hepatocellular proliferation and tumor angiogenesis. Lab Invest. 1999;79:225–234. [PubMed] [Google Scholar]
- 62.Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Lan-gley KE, Bahou WF, Docherty AJ, Cao J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase (MT1-MMP) J Biol Chem. 1998;273:1216–1222. doi: 10.1074/jbc.273.2.1216. [DOI] [PubMed] [Google Scholar]
- 63.Overall CM, Tam E, McQuibban GA, Morrison C, Wallon UM, Bigg HF, King AE, Roberts CR. Domain interactions in the gelatinase A.TIMP-2.MT1-MMP activation complex. The ectodomain of the 44-kDa form of membrane type-1 matrix metalloproteinase does not modulate gelatinase A activation. J Biol Chem. 2000;275:39497–39506. doi: 10.1074/jbc.M005932200. [DOI] [PubMed] [Google Scholar]
- 64.Hoegy SE, Oh HR, Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 (TIMP-2) suppresses TKR-growth factor signaling independent of metalloproteinase inhibition. J Biol Chem. 2001;276:3203–3214. doi: 10.1074/jbc.M008157200. [DOI] [PubMed] [Google Scholar]


