Abstract
Purpose
The aim of this analysis was to study whether monozygotic twinning (MZT) events occur in clusters after IVF and, if so, to explore possible explanations for this clustering.
Methods
This is a retrospective cohort study carried out in a single, large university-affiliated reproductive medicine practice. Medical records of all patients who had undergone fresh IVF cycles, resulting in a viable clinical pregnancy, from Jan 2002 to Dec 2013 were reviewed. The incidence of MZT in 6-month intervals and the association with independent risk factors such as maternal age, extended embryo culture, PGD/intracytoplasmic sperm injection (ICSI)/assisted hatching performed were analyzed.
Results
Over the 12-year study period, 25,502 fresh IVF cycles were performed, resulting in 8598 clinical pregnancies. Ninety-five cycles (1.1 %) resulted in MZ twins. The percentage of MZT was >2 standard deviations (SD) higher than the overall percentage of MZT in 4 of the 24 6-month intervals. PGD, extended embryo culture (≥4 days), and more recent cycles (2005 or later) were independent risk factors for MZT. The use of multivariable logistic regression modeling to control for risk factors for MZT did not correct for this clustering effect, with both high-risk interval (clustering) and extended embryo culture remaining significant.
Conclusion
This study supports our hypothesis that MZT occurs in clusters and that this clustering effect could not be explained by demographics and cycle characteristics alone. Although we are unable to explain the clustering phenomenon, this study is important as it highlights high-risk intervals for MZT and opens the door to performing a more detailed investigation, to identify the mechanisms responsible for the spikes of MZT incidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0616-x) contains supplementary material, which is available to authorized users.
Keywords: Monozygotic twins, In vitro fertilization, Risk factors, Trends
Introduction
The twin birth rate has increased by 76 % from 1980 through 2009, and now comprises 3 % of all US births [1]. Over this timeframe, there has been a shift in the demographic of women giving birth with a trend towards delayed childbearing. In 1980, women aged 30 and over accounted for about 20 % of all births compared with more than 35 % for 2000–2009 [1]. It is hypothesized that the shift to older childbearing explains about one third of the increase in twinning over the past 30 years, with assisted reproductive treatments accounting for the remainder. The study of multiple births is important because of their elevated health risks and accompanying greater health care costs [2].
Monozygotic (MZ) twins carry a significantly higher risk of perinatal morbidity and mortality than singleton and dizygotic twins [3, 4], including an increased risk of premature delivery [3], growth discordance [5], developmental anomalies [6], and mortality [3]. A proportion of these adverse events can be attributed to twin-to-twin transfusion syndrome, which complicates 10–20 % of all monochorionic twin pregnancies [7]. Furthermore, IVF gestations may, independently, have an increased risk of preterm delivery, placental abruption, low birth weight, preeclampsia, and perinatal mortality [8].
It is well recognized that the incidence of MZ twinning (MZT) is greatly increased among IVF patients compared to the general population (0.7–13 vs. 0.45 %, respectively), but the reason for this remains unclear [9, 10]. Current evidence suggests that multiple factors might be responsible, including maternal age, ovarian stimulation, prolonged embryo culture, altered in vitro culture conditions, and zona pellucida manipulation [11]. However, a meta-analysis and a recent large study did not show an association between intracytoplasmic sperm injection (ICSI) or assisted hatching and MZT [9, 12]. Blastocyst culture appears to be one of the most important factors contributing to MZ pregnancies [10, 11]. Knopman et al. [13] noted increased MZT rates among younger women when the maternal age of oocytes was <35 years; however, these results are confounded by the fact that younger women were more likely to have embryos transferred at the blastocyst stage [13]. While most studies suggest that blastocyst transfer is an independent risk factor for MZT [11–14], not all publications support this association [15]. For example, a recent study by Franasiak et al. [16] failed to demonstrate increased rates of MZT when controlling for embryo cohort quality. In that study, the authors also controlled for patient age, number, and proportion of six-to-eight cell embryos, as well as other factors. Studies have also hypothesized that media characteristics—such as glucose [17] or glutathione levels—may affect MZT rates. In addition, Sobek et al. [18] suggested that the high incidence of MZT in infertility patients is conditioned by hereditary factors and that good ovarian function only facilitates the expression of these factors, independent of the duration of embryo culture or the use of micromanipulation techniques [18].
To further complicate the discussion, a recent study by Osianlis et al. [19] estimated that as many as one in five twins born after single-embryo transfer may be the result of a concurrent natural conception. As such, couples need to be counseled about the risks of unprotected intercourse while undergoing fertility treatment.
Although the above studies confirm an association between MZT and different treatments offered during a routine IVF cycle, including blastocyst culture, the underlying mechanism remains unclear. We posit that an examination of the time course of MZT might pinpoint cycles when MZT was at the highest risk. Our primary hypothesis is that MZT events occur in clusters. If this hypothesis were correct, it would warrant further investigation into possible reasons behind this clustering. One possibility is that of external factors, such as the choice of embryo culture media. A better understanding of these external factors may aid us in identifying inciting factors and, potentially, provide an opportunity to modify such factors in an attempt to reduce MZT rates in assisted reproduction.
Methods
Subjects
A retrospective cohort study was performed using an established and validated IVF database from Boston IVF in Waltham, MA. Consecutive fresh IVF cycles resulting in a clinical pregnancy (defined as at least one gestational sac with a fetal pole and cardiac activity recorded on early ultrasound) from January 2002 to December 2013 were reviewed. All pregnancies, regardless of age and treatment type, achieved by the use of both autologous and heterologous (oocyte donation) in fresh cycles were included. Frozen embryo transfer cycles were excluded from the analysis to minimize variation and standardize the embryo cohort. Within this cohort, cases consisted of MZ pregnancies (as defined below) and controls consisted of all other pregnancies.
All pregnant patients underwent transvaginal ultrasound evaluation in the mid-first trimester. A MZ pregnancy was identified at that time when more than one fetal pole with cardiac activity was recorded in a single gestational sac or when the number of fetal poles with cardiac activity seen and recorded exceeded the number of embryos transferred. To confirm the MZ pregnancy, medical records were reviewed and demographic and clinical data abstracted, including subsequent ultrasound reports and maternal-fetal medicine consult reports.
Protocols
Patients underwent ovarian stimulation protocols with gonadotropins and either a GnRH agonist or antagonist as previously described [33]. Cycles were monitored with daily serum estradiol levels and transvaginal ultrasound examinations beginning on treatment days 6 to 8. When at least three follicles measured 15 to 20 mm, either 250 μg recombinant hCG (Ovidrel; EMD Serono) or 10,000 U of urinary hCG (Novarel; Ferring Pharmaceuticals) was administered subcutaneously. Ultrasound-guided oocyte retrieval was performed 36 h after hCG administration. During the period 2002 to 2013, embryos were cultured in a number of different types of embryo culture media, different protein supplements were used, and embryo transfer policy varied from day 3 to day 5 transfers. These changes are summarized in Fig. 1b.
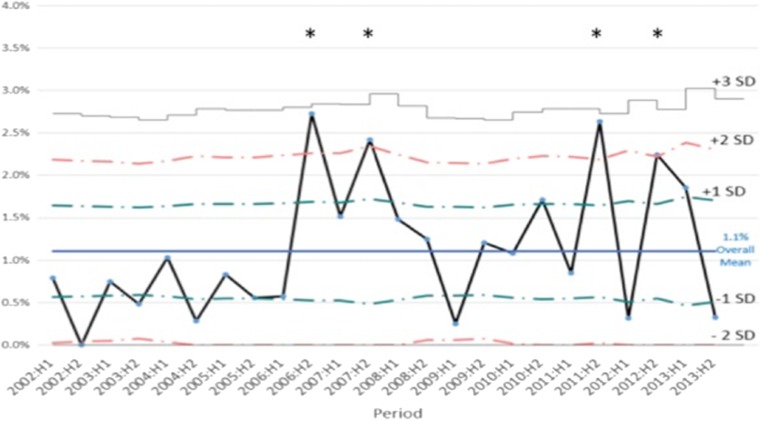
Fig. 1.
A U-chart displaying 6-month MZT rates over time with reference to standard deviation. High-risk intervals >2 SD are indicated by an asterisk
Embryos were transferred either on day 2/3 (cleavage stage) or day 5/6 (extended culture/blastocyst stage) on a case-by-case basis, per provider preference and institution protocol, although throughout the course of our study, there was a progressive trend towards increasing proportions of blastocyst transfers. Luteal support consisted of either progesterone vaginal cream one application daily (Crinone 8 %, Merck, Serono) or progesterone pessaries 200 mg three times per day (Prometrium, Catalent) or intramuscular progesterone 50 mg once daily.
Statistical analysis
We examined the event of clustering in several ways. A 6-month interval was chosen to look at clustering in relation to dates for the majority of analysis because of limitations in the number of cases. The incidence of MZT overall and separately for each 6-month interval was calculated as the total number of MZT events divided by the number of viable clinical pregnancies. Six-month intervals with a MZT incidence rate >2 standard deviations (SD) higher than the overall rate were regarded as a high-risk interval (i.e., cluster). An electronic flag was added to identify an IVF pregnancy occurring during the high-risk interval.
Categorical variables were compared between groups using chi-square and continuous variables with two sample t tests. Univariate analysis was used to identify factors associated with MZT. Variables with unadjusted associations with corresponding p values of p < 0.05 together with variables identified based on prior literature were included in the final multivariable logistic regression analysis model used to get estimates of the adjusted association of risk factors with MZT. The high-risk interval indicator was then added to the multivariable model to assess if the MZT could be explained by factors other than timing (i.e., event happening in a 6-month window [cluster]), after adjusting for other covariates.
We also examined the number of days between each MZT event and compared this to the interval expected if there was an even distribution of MZT events across the study period.
All statistical analyses were done using SAS for Windows, SAS 9.4 TS Level 1 M1 (SAS Institute Inc., Cary, NC, USA). A control chart was produced to display 6-month incident rates over time using the U-chart option of QI Macros for Excel (KnowWare International Inc.). For the statistical analyses, an α-error of <0.05 was considered significant.
This retrospective cohort study was a joint endeavor between Boston IVF/Beth Israel Deaconess Medical Center and Tufts Medical Center. The use of the de-identified database for the purpose of this analysis was deemed exempt by the Tufts Medical Center and Beth Israel Deaconess Medical Center Institutional Review Boards.
Results
All patients
There were a total of 25,502 fresh IVF cycles carried out at our institution between Jan 2002 and Dec 2013. Of these, 23,819 (93.4 %) were autologous and 1683 (6.6 %) were heterologous (donor/recipient) cycles. Of these cycles, 8598 (33.7 %) resulted in a viable clinical pregnancy. Table 1 illustrates the demographics and cycle characteristics of the autologous and heterologous fresh cycle over the study period. Of these pregnancies, 95 (1.1 %) resulted in MZT. The mean age of our autologous fresh cycle cohort was 36.4 years and the mean recipient age in the heterologous (oocyte recipient) group was 41.8 years. Of note, the anonymous donor age was <30 years in all cases.
Table 1.
Cycle characteristics of all fresh cycles performed between January 2002 and December 2013
| Fresh transfers | ||
|---|---|---|
| Homologous | Donors | |
| Number of cycles | 23,819 | 1683 |
| Age (years) | 36.4 ± 4.5 | – |
| Recipient age (years) | – | 41.8 ± 4.5 |
| Number of oocytes retrieved | 10.9 ± 6.7 | 17.4 ± 9.4 |
| Number of day 3 transfers | 20,248 | 1329 |
| (%) | (85.0 %) | (79.0 %) |
| Number of day 5 transfers | 3227 | 337 |
| (%) | (13.6 %) | (20.0 %) |
| Other days of transfer | 344 (1.4 %) |
17 (1.0 %) |
| Number of embryos transferred | 2.4 ± 1.2 | 2.0 ± 0.5 |
| Number of clinical pregnancies | 7856 | 742 |
| (%) | (33.0 %) | (44.1 %) |
| Cycles with ≥1 live birth | 6672 | 674 |
| (%) | (28.0 %) | (40.0 %) |
| Number of IVF | 14,194 | 938 |
| (%) | (59.6 %) | (55.7 %) |
| Number of intracytoplasmic sperm injection (ICSI) | 9625 | 745 |
| (%) | (40.4 %) | (44.3 %) |
| Preimplantation genetic screening/diagnosis (PGS/D) performed | 881 | 22 |
| (%) | (3.7 %) | (1.3 %) |
Data are provided as either the number of cases and percent or mean ± standard deviation (SD)
The number of oocytes retrieved in our autologous group was 10.9 ± 6.7, which, as expected, was less than the mean number of oocytes retrieved from the donors (17.4 ± 9.4). A total of 40.4 % (9625) patients in the autologous group underwent ICSI and 44.3 % (745) in the heterologous group. Three thousand two hundred twenty-seven (13.5 %) of the autologous cycles resulted in the blastocyst transfer of embryos, which was lower than that in the heterologous cycle group (337 [20 %]), consistent with superior embryo quality. The mean number of embryos transferred in the autologous cycle group was 2.4 ± 1.2, greater than in the heterologous group (2.0 ± 0.5). Eight hundred and eighty-one (3.7 %) of the autologous group underwent preimplantation genetic screening/diagnosis compared with only 22 women (1.3 %) in the donor oocyte recipient group. The number of clinical pregnancies in the autologous group was 7856 (33 %) and this resulted in 6672 (28 %) cycles with at least 1 live birth. In the heterologous cycle group, there were 742 (44.1 %) clinical pregnancies, resulting in 674 (40 %) cycles with at least 1 live birth.
Monozygotic twinning rates
Of all MZ pregnancies, 87/95 (91.6 %) resulted from autologous ART cycles with 8/95 (8.4 %) resulting from heterologous cycles. The rate of MZ clinical pregnancies was 88/7856 (1.1 %) among autologous cycles and 8/742 (1.1 %) among heterologous cycles. The mean age of the 95 cases in the MZ pregnancy cohort was 35.4 years and, on average, each cycle yielded 13.9 oocytes. Forty-three out of the ninety-five cases (45.3 %) were blastocyst transfers and, of these, 30/43 (69.8 %) had 2 or more blastocysts transferred. Table 2 highlights the demographic and cycle characteristics for the MZ pregnancies. Overall, the incidence of MZT among those who had blastocyst transfer was 2.7 % (43/1584), which was significantly greater than the incidence of MZ pregnancies among those who underwent day 2/3 transfer (0.7 % or 52/7014 clinical pregnancies) (p < 0.05). Seventy-nine out of ninety-five (83.2 %) resulted in at least one live birth. Following counseling, 8/95 (8.4 %) of women elected multifetal pregnancy reduction. Of these, 7/8 (87.5 %) resulted in one live birth. Thirty-seven out of ninety five (38.9 %) of cycles that resulted in a viable clinical pregnancy had some form of zona pellucida manipulation (Table 2).
Table 2.
Fresh monozygotic twin cycle characteristics
| Factor | Value |
|---|---|
| Age (years) | 35.4 ± 4.4 |
| Number of oocytes | 13.9 ± 7.5 |
| Number of mature oocytes | 11.8 ± 7.0 |
| Number of day 2/3 transfers | 52 (54.7 %) |
| Number of day 5 transfers | 43 (45.3 %) |
| Number of embryos transferred | 2.1 (0.9) |
| Zona pellucida manipulation | 37 (38.9 %) |
| Intracytoplasmic sperm injection | 30 (31.6 %) |
| Preimplantation genetic screening/diagnosis performed | 12 (12.6 %) |
| Assisted hatching | 7 (7.4 %) |
Data are provided as either the number of cases (n) and percent or mean ± standard deviation (SD)
MZT cases were compared with controls for cycle parameters (Table 3). Although the age of the oocyte donors was lower than that of the women undergoing autologous cycles, maternal age at cycle start was not an independent risk factor for MZT. There was no statistically significant difference between the risk of MZT in donor oocyte vs autologous cycles, or in the incidence of assisted hatching and the use of ICSI between the groups. However, we did note that extended embryo culture (≥4 days) significantly increased the risk of MZT (p < 0.0001) (Table 3). In contrast, increasing the number of embryos transferred decreased the risk of MZT (p = 0.015) likely because increasing numbers of embryos transferred are consistent with a day 2/3 transfer, rather than a blastocyst transfer. In addition, PGD appeared to be a significant risk factor for MZT in our univariate analysis (p < 0.0001). As our study period is prolonged and there have been both institutional as well as national changes in practice over this time frame, we found that the cycles performed in 2005 or later also increased the likelihood of a MZT (p = 0.003), which is consistent with an increasing proportion of blastocyst transfers.
Table 3.
Risk factors for MZT events among 8598 fresh IVF cycles from Jan 2002 through Dec 2013—N = 8598 pregnancies
| MZT (N = 95) |
Non-MZT (n = 8503) |
Unadjusted p valuea |
Adjusted OR (95 % CI) and p valueb |
|
|---|---|---|---|---|
| High-risk 6-month interval | 35.8 % (34) | 15.5 % (1321) | <.0001 |
2.37 (1.53, 3.66) p = .0001 |
| Embryo biopsy | 12.6 % (12) | 3.5 % (298) | <.0001 | 1.63 (0.82, 3.23) p = .16 |
| Assisted hatching | 7.4 % (7) | 11.1 % (940) | 0.2537 | – |
| Donor cycle | 8.4 % (8) | 8.7 % (743) | 0.9133 | – |
| ICSI | 31.6 % (30) | 38.5 % (3277) | 0.1655 | – |
| Year 2005 or later | 86.3 % (82) | 72.9 % (6197) | 0.0033 | 1.56 (0.84, 2.91) p = 0.16 |
| Extended culture | 45.3 % (43) | 18.1 % (1541) | <.0001 |
2.68 (1.66, 4.33) p < .0001 |
| Oocyte provider >35 years | 46.3 % (44) | 48.6 % (4136) | 0.6519 | – |
| Age at cycle start | 35.4 ± 4.4 | 35.9 ± 4.6 | 0.3753 | – |
| >2 embryos transferred | 20.0 % (19) | 31.7 % (2692) | 0.015 | 0.75 (0.45, 1.40) p = 0.42 |
Statistically signicant risk factors from the univariate analysis are presented in italics. These were included in the final multivariate model
aData shown as percent (n) with unadjusted p value from chi-square test, or as mean ± standard deviation with unadjusted p value from t test
bAdjusted odds ratios and p values from multivariable logistic regression
Clustering analysis of monozygotic twinning
As the primary aim of our study was to investigate clustering of MZT events in IVF, we divided our study period into 6-month intervals. Any period, in which the frequency of MZT was >2 SD higher than the overall average frequency of MZT across the entire study period, was flagged as a high-risk period. There were four such high-risk periods across the duration of the study: the second half of 2006, the second half of 2007, the second half of 2011, and the second half of 2012 (Fig. 1). We also examined the number of days between each MZT event and compared this to the interval expected if there was an even distribution of MZT events across the study period.
If MZT events occurred due to chance and were evenly distributed across the study period, one would anticipate a MZT event every 46.2 days. Therefore, 50 % of all MZT events would occur at an interval of less than 46.2 days apart. Interestingly, among our cohort, 65/95 (68 %) occurred less than 46.2 days apart. A z test was performed which confirmed that this was statistically significant (p = 0.0003), which serves to strengthen our hypothesis of clustering (see supplemental figure 1).
Distributions of risk factors for MZT and non-MZT events are summarized in Table 3 with corresponding unadjusted p values. Adjusted odds ratios together with adjusted p values for risk factors from a multivariable model and the outcome of MZT are also provided in Table 3. When we controlled for the relevant risk factors identified in the univariate model (cycle in 2005 or later, PGD performed, extended embryo culture, number of embryos transferred) as well as maternal age at cycle start, the flagged “high-risk” 6-month interval remained an independent risk factor for MZT (p < 0.0001). Extended embryo culture also remained an independent risk factor (p < 0.0001). We used the Akaike information criteria to assess the fit of our multivariate modeling, which indicated that our modeling was strongest when we included only those risk factors that were significant based on our univariate model. We also assessed whether the occurrence of a MZT pregnancy could be predicted based on the presence of a MZT pregnancy in a preceding IVF cycle; however, this predictive modeling was not statistically significant. It only became significant if there were at least four MZT pregnancies in the preceding 4-month interval. Since MZT is a rare event, the current dataset was underpowered to test this model.
Discussion
The incidence of MZT after ART is known to be significantly higher than the spontaneous incidence, but the mechanisms underlying this phenomenon are poorly understood [11]. The identification of a MZ pregnancy has a direct impact on antenatal care and obstetrical management, particularly when the pregnancy is monochorionic, which comprises approximately two thirds of all MZT. Monochorionic twin pregnancies are associated with a threefold increase in perinatal mortality and a tenfold increase in prenatally acquired neurological lesions when compared with dizygous twins [20]. Because of the increased complication rates as well as the increased healthcare costs [3–7, 20], it is important to decipher why the incidence of MZT is higher in the setting of ART and, subsequently, what can be done to reduce this incidence. Multi-fetal pregnancy reduction (MFPR) of a higher-order multiple pregnancy to a singleton or twin pregnancy is associated with improved perinatal outcomes [21]. In our cohort of patients with at least one MZT pair, eight women (8.4 %) opted for MFPR. Of note, seven out of the eight women went on to deliver at least one live infant.
In our retrospective study examining a large cohort at a single, urban, fertility center, MZT occurred at a rate of 1.1 % of all clinical pregnancies, which is on the lower side of what has been previously reported [9, 11–14]. Of note, only viable pregnancies (those with an identifiable fetal heartbeat) were included in our study. Without genetic fingerprinting of placental DNA using highly polymorphic marker analysis, which has become the gold standard for determining zygosity [22], the true incidence of MZT is impossible to accurately determine. In addition, the occurrence of spontaneous concurrent fertilization may not be as rare as initially thought [19], which may further confound our estimates. In our study, we adhered to strict inclusion criteria for determining monozygosity and sought to confirm the diagnosis by reviewing follow-up ultrasounds, where possible. Despite these efforts, it is possible that we may have missed MZ pregnancies that were either biochemical or never developed two fetal poles with cardiac activity.
A cluster is defined as an unusually high incidence of a particular disease or disorder occurring in close proximity in terms of both time and geography. Historical clusters have been used to identify the cause of a number of cancers including scrotal cancer among chimney sweeps in the 1770s and mesothelioma and lung cancer in asbestos workers in the 1960s [23]. In our study we have used a similar strategy to identify whether MZ twinning occurs in clusters. The scope of this study was to identify periods where the chances were significantly higher for a MZ twin to occur. Our future aim is to expand this analysis to multiple clinics to identify commonalities within clustering events.
Our findings support our primary hypothesis that, across the 12-year interval of the study, the clustering of MZT pregnancies was observed across four of the 24 6-month intervals, which were thereafter referred to as the four “high-risk intervals” (Fig. 1). The fact that the flag for a high-risk interval remained statistically significant even after adjusting for other risk factors indicates that the high MZT in those periods cannot be explained by the risk factors that we controlled for, including those found to be statistically significant in our cohort as well as other risk factors commonly quoted in the literature. Our future aim is to expand this analysis to multiple clinics to identify commonalities within clustering events.
There are several proposed theories to explain the occurrence of MZT. One theory is that the zona pellucida facilitates MZT by mechanical splitting or disrupting the inner cell mass during the process of blastocyst hatching [24]. Indeed, excessive thickening or hardening of the zona has been associated with twinning [24]. While the zona may be involved in some cases of MZT, it is unlikely to be the an exclusive mechanism since MZT has been demonstrated to occur also following transfer of zona-free blastocysts [25]. Over the past decade, there has been a continual shift towards blastocyst transfers, which now comprise the majority of embryo transfers in many countries including the USA and Australia. Cleavage stage transfers are generally reserved for older women, those with a poorer prognosis, and those with fewer embryos [12]. In our study, cycles performed in 2005 or later appeared to have a significantly increased risk of MZT compared to those cycles performed before 2005. When we controlled for extended embryo culture, this significance disappeared. Extended embryo culture appears to be one of the most important factors contributing to MZ pregnancies [10, 11], which may support the aforementioned theory that a breach of the integrity of the zona pellucida leads to a herniation of blastomeres and the splitting of the embryo [26]. This hypothesis led to concerns about ART procedures, which deliberately disrupt the zona pellucida, such as ICSI, assisted hatching, and PGD/PGS [12]. However, this concern has been refuted by several recent studies [9, 13, 16]. In our cohort, 38.9 % (37/95) of our MZT pregnancies had undergone zona pellucida manipulation, which was similar to our background rate of zona manipulation (44 % [11,221/25,502]). Assisted hatching, initially thought to be of benefit to improve pregnancy rates, has been largely disproven, even among poor prognosis patients [27]. Neither assisted hatching nor ICSI were significant risk factors for MZT in our cohort.
Of all the forms of zona manipulation examined, PGD appeared to be most strongly associated with MZT based on our univariate model. PGD is performed most often on embryos reaching the blastocyst stage, whereas it was previously performed almost exclusively on day 3. In the majority of clustering periods identified in this study, day 3 biopsy was being performed. We found an increased incidence of MZT among those pregnancies conceived after extended embryo culture and transfer. The natural thinning of the zona pellucida during the aging process was initially thought to confer additional risk of MZT with increasing maternal age [28]. In our study, however, maternal age did not appear to be a significant risk factor for MZT. Similarly, we found no significant difference in risk of MZT between autologous and donor oocyte cycles. Genetic researchers have reported a higher incidence of spontaneous MZT within certain families [29] and this may be true also in the ART population [18].
The limitations of our study include changes in practices over the extended 12-year study period. The study is also limited by the retrospective nature of its design, which inherently lends itself to treatment and information bias. We postulate that such biases should be distributed equally among exposed and unexposed groups. Interestingly, although ultrasonography quality has improved over the study period, we have not detected more MZT towards the end of the study period. As MZT is a rare event, a prospective trial would need to involve several large centers, with DNA fingerprinting to determine the true incidence of monozygosity. The strength of our study is that all treatments were conducted at a single large center with standardized protocols, which limit variations in practice.
To our knowledge, this is the first study to evaluate the clustering of MZ pregnancies in assisted reproduction. Changes in practice over the 12-year study period would affect clustering across all pregnancy types; however, the clustering of monozygotic pregnancies was found in isolation. We postulate that the biology of the embryos would be constant across the study period. As such, the most likely explanation for this MZT clustering is that it is due to external factors either clinical (such as type of stimulation) or laboratory (type or lots of cultured media, days in culture). One suspect that has been proposed is embryo culture media, which has been shown also to influence birth weight as well as other fetal characteristics [30]. Others have proposed that substrate type, also, can also influence birth weight [31]. We reviewed our laboratory records for changes in embryo culture media and supplements over the study period. Since January 2012, we have been using a single-step culture media. This media is supplemented with a serum protein substitute. Of note, the first two 6-month high risk periods identified in the study involved the same culture media/substrate combination (sequential culture media and protein supplement) whereas later high risk periods involved sequential culture media or single-step media and a different protein supplement. Additional studies are needed to determine whether the type of culture media affects MZT rates.
The cause of MZT still remains unanswered even though various theories have been proposed [32]. This study is novel as it provides evidence that clustering of MZT does indeed occur during IVF and that these incidences cluster during certain periods. As noted above, for other clustering studies, this is a first step to identifying commonalities and eventually a cause. Our study also serves to validate existing data regarding possible causes for monozygotic twinning such as blastocyst transfer [11–14], but these risk factors alone do not explain the clustering phenomenon. Although we are unable to explain the clustering phenomenon, this study is important as it highlights high-risk intervals for MZT and opens the door to performing a more detailed investigation, through a multicentered study, into both clinical and laboratory differences to identify the mechanisms responsible for the spikes of MZT incidence.
Electronic supplementary material
Bar chart denoting MZT events based on interval (days) between events. 68% of MZT events had an interval <46.2 days apart, which would be the expected interval if MZT events were uniformly distributed and 50% should have occurred. The “p value” for the z-test against a null of 50% is 0.0003. (GIF 77 kb)
Compliance with ethical standards
Funding
None
Conflict of interest
Ms. Ruthazer’s institution (Tufts Clinical and Translational Science Institute, Tufts University) was reimbursed for statistical support. Dr Penzias is a paid consultant for OvaScience and receives payment for lectures including service on speaker bureaus for Ferring Pharmaceuticals.
Footnotes
Capsule This study supports our hypothesis that MZT occurs in clusters and that this clustering effect could not be explained by demographics and cycle characteristics alone.
References
- 1.Martin JA, Hamilton BE, Osterman MJK. Three decades of twin births in the United States, 1980–2009. NCHS data brief, no 80. Hyattsville: National Center for Health Statistics; 2012. [PubMed]
- 2.The ESHRE Capri Workshop Group Multiple gestation pregnancy. Hum Reprod. 2000;15(8):1856–1864. doi: 10.1093/humrep/15.8.1856. [DOI] [PubMed] [Google Scholar]
- 3.Sebire NJ, Snijders RJ, Hughes K, et al. The hidden mortality of monochorionic twin pregnancies. Br J Obstet Gynaecol. 1997;104:1203. doi: 10.1111/j.1471-0528.1997.tb10948.x. [DOI] [PubMed] [Google Scholar]
- 4.Glinianaia SV, Obeysekera MA, Sturgiss S, Bell R. Stillbirth and neonatal mortality in monochorionic and dichorionic twins: a population based study. Hum Reprod. 2011;26:2549. doi: 10.1093/humrep/der213. [DOI] [PubMed] [Google Scholar]
- 5.Maier RF, Bailobreski B, Gross A, Vogel M, Dudenhausen JW, Obladen M. Acute and chronic fetal hypoxia in monochorionic and dichorionic twins. Obstet Gynecol. 1995;86:973–977. doi: 10.1016/0029-7844(95)00290-8. [DOI] [PubMed] [Google Scholar]
- 6.Honma Y, Minakami H, Eguchi Y, Uchida A, Izumi A, Sato I. Relation between hemoglobin discordance and adverse outcome in monochorionic twins. Acta Obstet Gynecol Scand. 1999;78:207–211. doi: 10.1080/j.1600-0412.1999.780307.x. [DOI] [PubMed] [Google Scholar]
- 7.Harkness UF, Crombleholme TM. Twin-twin transfusion syndrome: where do we go from here? Semin Perinatol. 2005;29:296–304. doi: 10.1053/j.semperi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Kalra SK, Molinaro TA. The association of in vitro fertilization and perinatal morbidity. Semin Reprod Med. 2008;26:423–435. doi: 10.1055/s-0028-1087108. [DOI] [PubMed] [Google Scholar]
- 9.Vitthala S, Gelbaya TA, Brison DR, Fitzgerald CT, Nardo LG. The risk of monozygotic twins after assisted reproductive technology: a systematic review and meta-analysis. Hum Reprod. 2009;15:45–55. doi: 10.1093/humupd/dmn045. [DOI] [PubMed] [Google Scholar]
- 10.Knopman JM, Krey LC, Oh C, Lee J, McCaffrey C, Noyes N. What makes them split? Identifying risk factors that lead to monozygotic twins after in vitro fertilization. Fertil Steril. 2014;102(1):82–89. doi: 10.1016/j.fertnstert.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Aston KL, Peterson CM, Carrell DT. Monozygotic twinning associated with assisted reproductive technologies: a review. Reproduction. 2008;136:377–386. doi: 10.1530/REP-08-0206. [DOI] [PubMed] [Google Scholar]
- 12.Kawachiya S, Bodri D, Shimada N, Kato K, Takehara Y, Kato O. Blastocyst culture is associated with an elevated incidence of monozygotic twinning after single embryo transfer. Fertil Steril. 2011;95:2140–2142. doi: 10.1016/j.fertnstert.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Knopman J, Krey LC, Lee J, Fino ME, Novetsky AP, Noyes N. Monozygotic twinning: an eight-year experience at a large IVF center. Fertil Steril. 2010;94:502–510. doi: 10.1016/j.fertnstert.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 14.Sills ES, Moomjy M, Zaninovic N, Veeck LL, McGee M, Palermo GD, et al. Human zona pellucida micromanipulation and monozygotic twinning frequency after IVF. Hum Reprod. 2000;15(4):890–895. doi: 10.1093/humrep/15.4.890. [DOI] [PubMed] [Google Scholar]
- 15.Papanikolaou EF, Fatemu H, Venetis C, Donoso P, Kolibianakis E, Tournaye H, et al. Monozygotic twinning is not increased after single blastocyst transfer compared with singe cleavage-stage embryo transfer. Fertil Steril. 2010;93:592–597. doi: 10.1016/j.fertnstert.2008.12.088. [DOI] [PubMed] [Google Scholar]
- 16.Franasiak JM, Dondik Y, Molinaro TA, Hong KH, Forman EJ, Werner MD, et al. Blastocyst transfer is not associated with increased rates of monozygotic twins when controlling for embryo cohort quality. Fertil Steril. 2015;103:95–100. doi: 10.1016/j.fertnstert.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Menezo YJR, Sakkas D. Monozygotic twins: is it related to apoptosis in the embryo? Hum Reprod. 2002;17:247–251. doi: 10.1093/humrep/17.1.247. [DOI] [PubMed] [Google Scholar]
- 18.Sobek A, Jr, Zbořilová B, Procházka M, Šilhánová E, Koutná O, Klásková E, et al. High incidence of monozygotic twinning after assisted reproduction is related to genetic information, but not to assisted reproduction technology itself. Fertil Steril. 2015;103(3):756–760. doi: 10.1016/j.fertnstert.2014.12.098. [DOI] [PubMed] [Google Scholar]
- 19.Osianlis T, Rombauts L, Gabbe M, Motteram C, Vollenhoven B. Incidence and zygosity of twin births following transfers using a single fresh or frozen embryo. Hum Reprod. 2014;29:1438–1443. doi: 10.1093/humrep/deu064. [DOI] [PubMed] [Google Scholar]
- 20.Hillman SC, Morris RK, Kilby MD. Co-twin prognosis after single fetal death: a systematic review and meta-analysis. Obstet Gynecol. 2011;118:928–940. doi: 10.1097/AOG.0b013e31822f129d. [DOI] [PubMed] [Google Scholar]
- 21.Evans MI, Berkowitz RL, Wapner RJ, Carpenter RJ, Goldberg JD, Ayoub MA, et al. Improvement in outcomes of multifetal pregnancy reduction with increased experience. Am J Obstet Gynecol. 2001;184:97–103. doi: 10.1067/mob.2001.108074. [DOI] [PubMed] [Google Scholar]
- 22.Guilherme R, Drunat S, Delezoide AL, Le Ray C, Oury JF, Luton D. Zygosity and chorionicity in triplet pregnancies: new data. Hum Reprod. 2009;24:100–105. doi: 10.1093/humrep/den364. [DOI] [PubMed] [Google Scholar]
- 23.Thun MJ, Sinks T. Understanding cancer clusters. CA Cancer J Clin. 2004;54(5):273–280. doi: 10.3322/canjclin.54.5.273. [DOI] [PubMed] [Google Scholar]
- 24.Edwards RG, Mettler L, Walters DE. Identical twins and in vitro fertilization. J In Vitro Fert Embryo Transf. 1986;3:114–117. doi: 10.1007/BF01139357. [DOI] [PubMed] [Google Scholar]
- 25.Frankfurter D, Hackett R, Meng L, Keefe DL. Complete removal of the zona pellucida by pronase digestion prior to blastocyst embryo transfer does not eliminate monozygotic pregnancies following IVF. Fertil Steril. 2001;76:S144. doi: 10.1016/S0015-0282(01)02422-0. [DOI] [Google Scholar]
- 26.Alikani M, Noyes N, Cohen J, Rosenwaks Z. Monozygotic twinning in the human is associated with the zona pellucida architecture. Hum Reprod. 1994;9:1318–1321. doi: 10.1093/oxfordjournals.humrep.a138701. [DOI] [PubMed] [Google Scholar]
- 27.Kissin DM, Kawwass KF, Monsour M, Boulet SL, Session DR, Jamieson DJ, et al. Assisted hatching: trends and pregnancy outcomes, United States, 2000-2010. Fertil Steril. 2014;102(3):795–801. doi: 10.1016/j.fertnstert.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garside WT, De Mola J, Bucci JA, Tureck W, Heyner S. Sequential analysis of zona thickness during in vitro culture of human zygote: correlation with embryo quality, age, and implantation. Mol Reprod Dev. 1997;47:99–104. doi: 10.1002/(SICI)1098-2795(199705)47:1<99::AID-MRD13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Machin G. Familial monozygotic twinning: a report of seven pedigrees. Am J Med Genet. 2009;151C:152–154. doi: 10.1002/ajmg.c.30211. [DOI] [PubMed] [Google Scholar]
- 30.Eskild A, Monkerud L, Tanbo T. Birthweight and placental weight: do changes in culture media used for IVF matter? Comparisons with spontaneous pregnancies in the corresponding time periods. Hum Reprod. 2013;28(12):3207–3214. doi: 10.1093/humrep/det376. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Li M, Chen L, Liu P, Qiao J. The protein source in embryo culture media influences birthweight: a comparative study between G1 v5 and G1-PLUS v5. Hum Reprod. 2014;29(7):1387–1392. doi: 10.1093/humrep/deu103. [DOI] [PubMed] [Google Scholar]
- 32.Menezo Y, Sakkas D. Monozygotic twinning: is it related to apoptosis in the embryo? Hum Reprod. 2002;17(1):247–248. doi: 10.1093/humrep/17.1.247. [DOI] [PubMed] [Google Scholar]
- 33.Eaton JL, Hacker MR, Harris D, Thornton KL, Penzias AS. Assessment of day-3 morphology and euploidy for individual chromosomes in embryos that develop to the blastocyst stage. Fertil Steril. 2009;91:2432–2436. doi: 10.1016/j.fertnstert.2008.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bar chart denoting MZT events based on interval (days) between events. 68% of MZT events had an interval <46.2 days apart, which would be the expected interval if MZT events were uniformly distributed and 50% should have occurred. The “p value” for the z-test against a null of 50% is 0.0003. (GIF 77 kb)