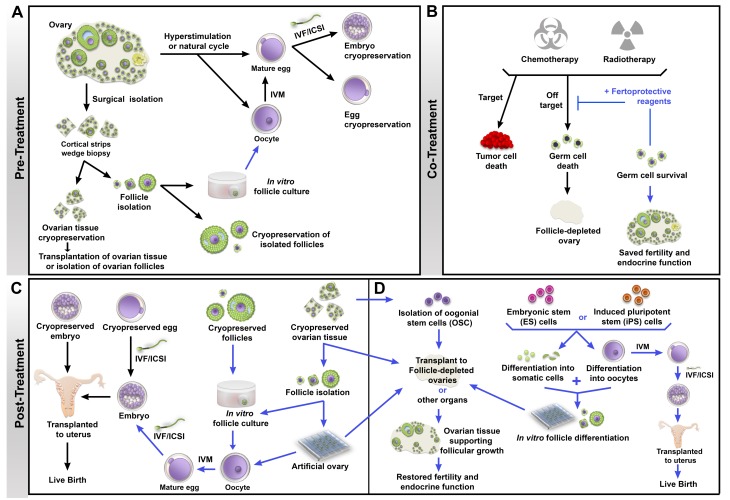
Fig. 2.
Schema of fertility preservation approaches in cancer patients. Each procedure is marked with a line, either black (established) or blue (investigational). (A) Patients undergoing a natural or hyperstimulated cycle produce mature eggs that can be matured in vitro (IVM) and either cryopreserved or fertilized by in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) to produce embryos that are cryopreserved. In patients who cannot undergo controlled ovarian stimulation (e.g., prepubertal girls or women with hormone-sensitive cancers) or in those who must start cancer treatment immediately, strips of ovarian cortex can be removed and cryopreserved, or individual follicles can be isolated from the tissues and cryopreserved. Investigational methods are focusing on use of thawed ovarian tissues for transplantation or in vitro growth of follicles, followed by IVM and IVF/ICSI. (B) During treatment, gonadotoxicity can be mitigated by the use of fertoprotective reagents that induce death in tumor cells while preventing off-target effects in other tissues, resulting in preserved fertility and endocrine function. (C) After cancer treatment, cryopreserved embryos can be thawed and transferred into the uterus, or cryopreserved eggs can be thawed and used for IVF/ICSI and the resulting embryos transferred to the patient. On the investigational side (blue arrows), cryopreserved follicles can be grown in culture, matured in vitro, and fertilized to produce embryos for transfer. Ovarian tissue can be transplanted back into the patient, or follicles can be isolated and used to produce embryos or cultured on a three-dimensional bioplotted scaffold as an artificial ovary for transplantation back into patients. Transplantation of ovarian tissue or follicles within an artificial ovary has the potential of restoring fertility as well as endocrine function after cancer treatment. (D) In patients who were unable to cryopreserve eggs, embryos, or ovarian tissue prior to treatment, researchers are now investigating the possibility of using oogonial stem cells (OSCs) to repopulate follicle-depleted ovaries, or differentiating follicle somatic cells and oocytes from embryonic stem (ES) cells or induced pluripotent stem (iPS) cells to assemble follicles de novo for transplantation or IVM and IVF/ICSI to create embryos for transfer.