Summary
Shigella dysenteriae causes the most severe of all infectious diarrhoeas and colitis. We infected rhesus macaques orally and also treated them orally with a small and non‐absorbable polypropyletherimine dendrimer glucosamine that is a Toll‐like receptor‐4 (TLR4) antagonist. Antibiotics were not given for this life‐threatening infection. Six days later, the clinical score for diarrhoea, mucus and blood was 54% lower, colon interleukin‐8 and interleukin‐6 were both 77% lower, and colon neutrophil infiltration was 75% less. Strikingly, vasculitis did not occur and tissue fibrin thrombi were reduced by 67%. There was no clinical toxicity or adverse effect of dendrimer glucosamine on systemic immunity. This is the first report in non‐human primates of the therapeutic efficacy of a small and orally bioavailable TLR antagonist in severe infection. Our results show that an oral TLR4 antagonist can enable controlled resolution of the infection‐related‐inflammatory response and can also prevent neutrophil‐mediated gut wall necrosis in severe infectious diarrhoeas.
Keywords: bacterial diarrhoea, cytokines, inflammation, Toll‐like receptor‐4
Abbreviations
- AFRIMS
Armed Forces Research Institute of Medical Sciences
- CFUs
colony‐forming units
- DG
dendrimer glucosamine
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- IFN‐γ
interferon‐γ
- IL‐6
interleukin‐6
- LPS
lipopolysaccharide
- MD‐2
myeloid differentiation factor 2
- NHP
non‐human primate
- PE
phycoerythrin
- RT‐PCR
reverse transcription–polymerase chain reaction
- TGF‐β
transforming growth factor‐β
- TIR
Toll/interleukin‐1 receptor
- TLR4
Toll‐like reeptor 4
- TNF‐α
tumour necrosis factor‐α
- TRAM
TRIF‐related adaptor molecule
- TRIF
TIR domain‐containing adapter‐inducing interferon‐β
Introduction
Global antimicrobial resistance means that alternatives to antibiotics are urgently needed. Invasive gut Gram‐negative bacteria (e.g. enteropathogenic/enterohaemorrhagic Escherichia coli, non‐typhoidal Salmonella, Shigella)1, 2, 3 and necrotizing enterocolitis in infants4, 5 cause life‐threatening disease by stimulating intense Toll‐like receptor 4 (TLR4) –myeloid differentiation factor 2 (MD‐2) –lipopolysaccharide (LPS) cytokine‐induced and neutrophil‐mediated gut wall damage.6 This provides pathogens with a substantial growth advantage and enhances their tissue invasion by destabilizing tight junctions, destroying gut mucosa and allowing transepithelial neutrophil migration into the gut lumen.7
Clinical trials targeting infection‐related‐inflammation fail because of inadequate mouse disease models for testing drugs.8 This is because mice are 6 log orders of magnitude more resistant to LPS‐induced septic shock than humans.8 In severe shigellosis however, human disease pathogenesis has been well defined9, 10 and we and others have recently described clinically validated non‐human primate (NHP) models for vaccine and drug testing.11, 12, 13 Cytokine‐mediated‐inflammation leads to excessive neutrophil infiltration and vasculitis of the colon.9, 10, 11, 14, 15 Clinically, this presents as bloody diarrhoea. Lack of effective vaccines for these severe infective diarrhoeas and the accelerating global antimicrobial resistance crisis make this a military and World Health Organization public health priority area.
This led us to propose that blocking the myeloid differentiation primary response gene 88 (MYD88) pathway of the TLR4‐MD‐2‐LPS‐mediated cytokine storm without interfering with the Toll/interleukin‐1 receptor (TIR) domain‐containing adapter‐inducing interferon‐β (TRIF)/TRIF‐related adaptor molecule (TRAM) pathway should prevent gut wall damage.16, 17, 18, 19, 20, 21 We have previously modelled, synthesized and tested a 13 600 MW polyamidoamine dendrimer glucosamine and a 75% smaller 3300 MW polypropyletherimine dendrimer glucosamine (DG) in a rabbit closed intestinal loop model of Shigella flexneri infection. No antibiotics were given. For these in vivo studies, the abdominal wall was opened and 5‐cm intestinal loops of gut were created into which S. flexneri bacteria and DG were injected. The abdomen was then closed and the rabbits were starved and monitored for 18 hr before autopsy. We showed a large reduction in colon interleukin‐6 (IL‐6) and IL‐8 and in Shigella invasion of tissues, with complete gut wall protection.22, 23, 24
Small dendrimer drugs are showing increasing promise as new polyvalent medicines in several animal models of infection and inflammation.21 Polypropyletherimine DG is a Good Manufacturing Process‐synthesized and analytically characterized generation 3 partially glycosylated dendrimer.21, 22, 23 It blocks the production of pro‐inflammatory cytokines by interfering with the electrostatic binding of: (i) the 4′ phosphate on the di‐glucosamine of LPS to Ser118 on MD‐2; (ii) LPS to Lys91 on MD‐2; and (iii) the subsequent binding of TLR4 to Tyr102 on MD‐2.22 Importantly, the human MD‐2 residues 118, 91 and 102 are conserved in rhesus macaque and this means that NHPs are as sensitive as humans to LPS.22, 25 DG is not toxic when given orally, intraperitoneally or intravenously to mice and rabbits in the dose range 0·1–175 mg/kg.23
We now describe the results of a Good Manufacturing Process and Good Laboratory Practice prospectively randomized study that fulfils US Food & Drugs Administration requirements for testing DG in rhesus macaques infected with 2 × 109 colony‐forming units (CFUs) of toxin‐producing Shigella dysenteriae Type 1. This bacterial pathogen causes the most severe of all infection and toxin‐related diarrhoeas and colitis. Antibiotics were not used. DG has no antibacterial activity against either Gram‐negative or Gram‐positive bacteria.
Materials and methods
Pharmaceutical grade synthesis of Good Manufacturing Process grade DG and its detailed analytical chemistry were performed at GlycoSyn (Lower Hutt, New Zealand).23 The Good Laboratory Practice NHP study and tissue analysis were performed at the Armed Forces Research Institute of Medical Sciences (AFRIMS; Bangkok, Thailand).12 NHP sample size was based upon our previous studies.12, 23
Non‐human primates
Adult rhesus macaques (Macaca mulatta; male and female, 5–15 years, weight 6·5–12 kg) of Indian origin were used. Animals were born and housed at AFRIMS and maintained according to the Guide for the Care and Use of Laboratory Animals, NRC, 2011. They were fed monkey feed (083G; Perfect Companion Co, Bangkok, Thailand), 15–20 biscuits daily and one or two treat tablets weekly. Fresh fruits and vegetables and hyper‐chlorinated water were also provided.
Study ethics
The NHP studies were performed under Institutional Animal Care and Use Committee (IACUC) protocol #PN 13‐05 ‘Defining the therapeutic efficacy of polypropyletherimine dendrimer glucosamine, a non‐antibiotic based drug, in rhesus monkeys after Shigella dysenteriae infection’, which was reviewed and approved by the AFRIMS IACUC and the Biosafety Review Committee. The AFRIMS‐Primate Research Committee is fully accredited by the Association for Assessment & Accreditation of Laboratory Animal Care International. Studies were conducted in compliance with the Animal Welfare Act and all other US federal statutes and regulations.
NHP pre‐study screening
All NHPs had negative serological test results for simian immunodeficiency virus, simian retrovirus, simian T‐cell leukaemia virus‐1 and were also negative for tuberculosis. On day −35 before S. dysenteriae Type 1 infection, 40 NHPs were moved to a separate wing and treated with azithromycin for 5 days as a prophylactic measure. Rectal swabs and stool swabs were taken to confirm the absence of enteric pathogens. On day −14, the AFRIMS biostatistician randomized a subgroup of 18 NHPs using the nquery advisor v. 6·01 procedure (i.e. random subset of cases and basic randomization list), and they were moved to an Animal Biological Safety Level 3 suite in individual cages. Before day 1 (defined as the day of infection), any NHP that did not meet all of the inclusion criteria was replaced. On day −40 and again on day −14, NHPs were screened by physical examination, complete blood count and chemistry, and stool culture with a rectal swab, and gingival swab for enteric pathogens including Shigella, Salmonella and Campylobacter spp., and plasma anti‐S. dysenteriae Type 1 LPS‐specific antibody (IgA, IgG and IgM) titres. Although it is common for NHPs to have subclinical Campylobacter spp. in their gut, only those NHPs that were free of diarrhoea and Campylobacter spp. on day −14 were allowed to progress; 14 NHPs finally entered the infection/treatment part of the study.
Study design
The characteristics of the master cell bank of the S. dysenteriae Type 1 1617 strain26 used were confirmed by (i) slide agglutination test to show it agglutinated with S. dysenteriae Type 1 specific sera; (ii) plaque assay to show that it invaded intracellularly by forming hollow plaques in LLC‐MK2 cells; (iii) stability assay to show that it was stable by having > 80% of single‐well‐isolated virulent colonies present after three consecutive sub‐cultures on Congo Red agar plates. Our previous studies have determined that the optimal challenge dose for an 80% attack rate of shigellosis is 2 × 109 CFUs.12 On day 1, NHPs were challenged with S. dysenteriae 4 hr after the first dose of DG. The bacterial suspension was given directly into the stomach using a paediatric nasogastric tube in a dose of 2 × 109 CFU in 20 ml sterile PBS and after 20 ml bicarbonate buffer to neutralize stomach acid. The DG was given (dissolved in sterile water at 6 mg/kg) once daily, and into the stomach using a nasogastric tube after 20 ml bicarbonate buffer on days 1, 2, 3, 4 and 5. Control NHPs received 20 ml sterile water into the stomach via a nasogastric tube and after 20 ml bicarbonate buffer. After each dose of DG, NHPs were monitored for 1 hr for any adverse events. Stool was collected twice daily for culture and PCR. Isolation of the S. dysenteriae Type 1 1617 challenge strain from stool was confirmed by slide agglutination and real‐time PCR for ipaH. Stool S. dysenteriae CFU was not quantified.
The primers and probes used were:
-
ipaH‐U1 (f)CCTTTTCCGCGTTCCTTGA
& ipaH‐L1 (r)CGGAATCCGGAGGTATTGC.
ipaH‐P1 probe VIC‐CGCCTTTCCGATACCGTCTCTGCA‐TAMRA.
-
OOK1 (f)AGGAATCCATTTGTGTACCAAATGA
& OOK2 (r)AACATGAGCACATTGGAATTTTAGC.
PGAL‐P probe VIC‐TGAAAGTGGGAAATGTGTACTAGATCCAAAACAAGA‐TAMRA (internal control).
The vet (RI) was allowed to rehydrate NHPs by subcutaneous infusion with water, sodium and potassium if they became clinically dehydrated. No antibiotics were given after infection with S. dysenteriae. Neither Shigella‐infected nor DG‐treated NHPs lost > 5% body weight. On day 6, NHPs had a whole body autopsy with all tissue samples collected within 30 min of death.
NHP monitoring
They were scored daily for presence {1} or absence {0} of each of diarrhoea, mucus and blood in stool to define the % clinical score. Haemoglobin, white blood cell count, cell type differential, platelets, urea, creatinine, albumin, bilirubin, alanine aminotransferase and γ‐glutamyltransferase were monitored daily.
Oral dosing of DG
Studies have shown a gut transit time of ~ 16 hr so a once‐daily oral dosing regimen was used. Three of the NHPs treated had a low body weight of 7·13 ± 0·39 kg/NHP; they received a total study DG dose of 214 ± 12 mg/NHP. They are referred to as the DG (214 mg) group. Three of the NHPs treated had a high body weight of 11·13 ± 0·72 kg/NHP; they received a total study DG dose of 334 ± 21 mg/NHP. They are referred to as the DG (334 mg) group.
Quantitative mRNA RT‐PCR
Dissected tissue was collected in RNALater and disrupted with a bead beater. RNA was extracted using an RNeasy mini kit (Qiagen, Hilden, Germany). Quantitative one‐step real‐time RT‐PCR27, 28, 29 was performed for IL‐1β, IL‐6, IL‐8, IL‐10, tumour necrosis factor‐α (TNF‐α), interferon‐γ (IFN‐γ), FoxP3 and transforming growth factor‐β (TGF‐β) using the primer pairs and probes shown below. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as the housekeeping gene.23 Positive control recombinant plasmids were provided by Francois Villinger (Emory University, Atlanta, GA). Probes were 5’‐labelled with FAM (6‐carboxyfluorescein) and 3’‐labelled with Black Hole Quencher except for GAPDH, which was 5’‐labelled with Cal Fluor Orange 560 and 3’‐labelled with Black Hole Quencher. Each tissue sample was analysed twice.
Primer pairs & probes (5’ to 3’) used for quantitative RT‐PCR
-
IL‐1 β : (f)GAGCAACAAGTGGTGTTCTCCA and (r)TCTTTCAACACGCAGGACAGG.
Probe CAAAATACCTGTGGCCTTGGGCCTCAA.
-
IL‐6: (f)TGGCTGAAAAAGATGGATGCT and (r)TTGCTCCTCACTACTCTCAAACCT.
Probe TGATTTTCACCAGGCAAGTGTCCTCATTG.28
-
IL‐8 [CXCL8}: (f)TGGCTCTCTTGGCAGCCT and (r)TTGGGGTGGAAAGGTTTGGA.
Probe TCTGTGAAGGTGCAGTTTTGCCAAGG.30
-
IL‐10: (f)GAGAACCACGACCCAGACAT and (r)ATTCTTCACCTGCTCCACGGC.
Probe CCTGAGGGTCTTCAGATTCTCCCCCAG.31
-
TNF‐ α : (f)GGCTCAGGCAGTCAGATCATC and (r)GCTTGAGGGTTTGCTACAACATG.
Probe TCGAACCCCAAGTGACAAGCCTGTAGC.28
-
IFN‐ γ : (f)GAAAAGCTGACCAATTATTCGGTAA and (r)AGCCATCACTTGGATGAGTTCA.
Probe TGACTCGAATGTCCAACGCAAAGCAGTA.28
-
FoxP3: (f)CACCTGGCTGGGAAAATGG and (r)GCAGGAGCCCTTGTCAGATG.
Probe CACTGACCAAGGCTTCATCTGTGGCAT.32
-
TGF‐ β : (f)GTATATTGACTTCCGCAAGGACCT and (r)TGTCCAGGCTCCAAATGTAGG.
Probe AGGGCTACCATGCCAACTTCTGCCTG.
-
GAPDH: (f)GCACCACCAACTGCTTAGCAC and (r)TCTTCTGGGTGGCAGTGATG.
Probe TCGTGGAAGGACTCATGACCACAGTCC.28
Histology
Tissue samples were collected in 10% neutral‐buffered formalin, processed routinely, and stained with haematoxylin & eosin. All slides were coded and interpreted and scored blind by EL (a Board certified Diplomate of the American College of Veterinary Pathologists) for: (i) Tissue inflammation33 – within normal limits = 0; minimal = 1; mild = 2; moderate = 3; marked = 4; severe = 5; (ii) Cell type infiltrate (i.e. neutrophil, lymphocyte, plasma cells, monocyte, eosinophil)34 – absent = 0 or present = 1; (iii) Vasculitis – absent = 0 or present = 1; and (iv) Fibrin thrombi35 – absent = 0 or present = 1.
Flow cytometry
Blood was collected into BD Vacutainer CPT cell preparation tubes with sodium citrate and into BD Vacutainer sodium heparin tubes. Plasma and buffy coat layers were harvested and frozen. Surface staining was performed for CD3 [allophycocyanin‐conjugated mouse anti‐human CD3 (clone SP34‐2) #557597]; CD4 [FITC‐conjugated mouse anti‐human CD4 (clone M‐T477) #556615]; CD8 [Peridinin chlorophyll protein‐conjugated mouse anti‐human CD8 (clone SK1) #347314]; CD14 [FITC‐conjugated mouse anti‐human CD14 (clone M5E2) #557153]; Ki67 [FITC‐conjugated mouse anti‐human Ki‐67 (clone B56) #561283] (BD Pharmingen, San Diego, CA).
Intracellular staining was performed for TNF‐α [phycoerythrin [PE] ‐conjugated mouse anti‐human TNF‐α (clone MAb11) #557068]; IL‐1β [PE‐conjugated mouse anti‐human IL‐1β (clone AS10) #340516]; IL‐6 [PE‐conjugated rat anti‐human IL‐6 (clone MQ2‐6A3) #551473]; IL‐8 [PE‐conjugated mouse anti‐human IL‐8 (clone G256‐8) #554720]; (all BD Pharmingen) before and after stimulation with PMA (50 ng/ml), ionomycin (5 μg/ml) and GolgiPlug containing Brefeldin‐A (10 μg/ml).
Chopped tissue from each of ascending colon, transverse colon and descending colon, and from rectum and mesenteric lymph nodes was treated with EDTA‐dithiothreitol, digested with collagenase, and the collected cell suspension was enriched for lymphocytes. Surface staining was performed for CD3, CD4, CD8, Ki67 and T‐cell receptor γδ (TCR‐γδ) [FITC‐conjugated mouse anti‐human TCR‐γδ (clone B1) #559878; BD Pharmingen]. A flow cytometer‐FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) equipped with automated FACS Loader & cellquest Software was used for data acquisition and analysis as previously described.36
Data and statistical analyses
Data were analysed using graphad prism 6 software (GraphPad, San Diego, CA) and a two‐tailed non‐parametric Mann–Whitney test. Results are shown as mean ± SEM with *P < 0·05, **P < 0·01, ***P < 0·001.
Of the six rhesus macaques infected and treated with placebo, one was excluded because it was not infected: (1) it never required rehydration; (2) after 5 days was still eating biscuits (i.e. no anorexia); (3) stool was normal in form and consistency; (4) neither blood nor mucus was present in stool; and at autopsy (4) entire gastrointestinal tract was normal; (5) background inflammation was only found on histology of jejunum, ileum, colon and rectum; and (6) IL‐8, IL‐6 and IL‐1β in ascending, transverse and descending colon, and rectum, were the same as in normal NHPs.
Death from fulminant dysentery (i.e. bloody diarrhoea) is well recognized in the rhesus macaque model of Shigella infection.12, 14, 37 One of the NHPs infected and treated with DG was excluded because: (i) it developed severe septic shock soon after infection; (ii) it required early and considerable subcutaneous fluid rehydration; (iii) autopsy was 1 day early because of clinical deterioration and distress; and at autopsy (iv) a Waterhouse–Friderichsen syndrome was suggested by the histology in association with an enlarged, cavitating and necrotizing adrenal gland;38, 39 and there were (v) severe and segmental necro‐haemorrhagic jejunitis, ileitis and typhlitis; (vi) diffuse, severe, neutrophilic colitis with focal necrotizing vasculitis; and (vii) massive colon cytokine storm with IL‐8 1200% higher, IL‐6 1120% higher and IL‐1β 300% higher than in the Shigella infection controls.
Results
Clinical outcome and score
In two NHPs, all of the procedures were performed and they were treated with water for injection only; they are the normal group. Six NHPs were infected with S. dysenteriae and they were treated with water for injection only; they are the Shigella group. Six NHPs were infected with S. dysenteriae and were treated with oral DG once daily for 5 days; they are the DG group. Autopsy was on day 6. The total study dose of DG was either 214 ± 12 mg/NHP (n = 3) or 334 ± 21 mg/NHP (n = 3) (P = 0·02).
Time to positive Shigella stool culture/PCR was 1·6 ± 0·2 days for all three groups. Infection led to a white cell count of 11·6 ± 1·2 compared with 7·9 ± 1·0 × 103/μl (P = 0·04) in the normal group. Blood albumin fell from 4·36 ± 0·07 (normal group) to 2·76 ± 0·3 g/dl (Shigella group) (P = 0·002) because primates develop a protein‐losing enteropathy.40 No clinical toxicity of DG was seen, and DG had no effect on any haematological or biochemical parameter (data not shown).
Colon histopathology score
Treatment with DG (334 mg) for 5 days reduced the clinical score for diarrhoea, mucus and blood on day 6 by 54% (Fig. 1a). At the time of autopsy, the colon was macroscopically assessed for evidence of acute gut wall tissue injury; representative examples are shown in Fig. 2. All histological tissue sections were scored blind by EL (a Board certified Diplomate of the American College of Veterinary Pathologists). DG reduced the colon inflammation score by 71% (Fig. 1b). This was the result of a 75% reduction in gut wall infiltration by neutrophils with no significant change in either monocyte or lymphocyte infiltration (Figs 1c and 3). The notable absence of tissue necrosis with DG treatment suggests that Shigella's reprogramming of neutrophils to die by necrosis (with inflammation) instead of apoptosis (without inflammation) did not occur. This enabled controlled resolution of the infection‐related‐inflammatory response.23, 41, 42, 43, 44
Figure 1.
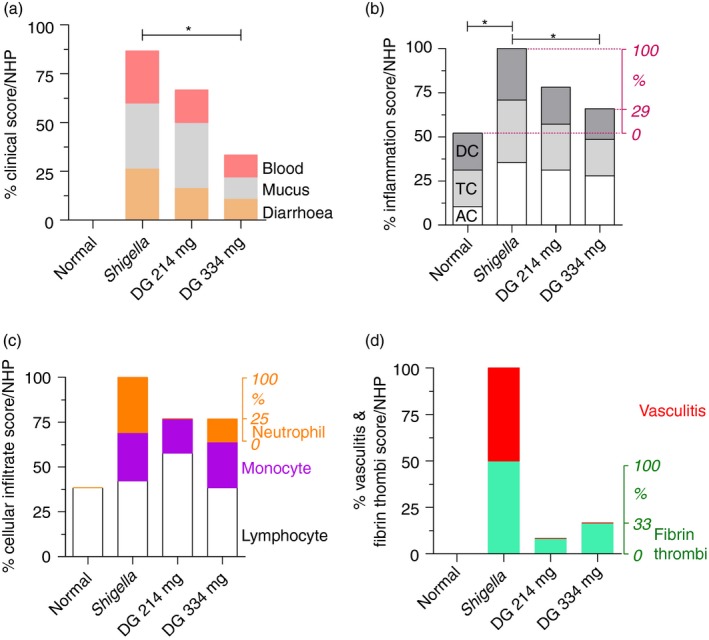
Clinical score and colon histology score. (a) Dendrimer glucosamine (DG) (334 mg) reduced the total clinical score for diarrhoea, mucus and blood/non‐human primate (NHP) by 54%. (b) The right side y‐axis shows the % inflammation score/NHP after correcting for the % inflammation score/NHP being 52% in normal NHPs as shown on the left side y‐axis. DG (334 mg) reduced the colon inflammation score/NHP by 71%. (c) The right side y‐axis shows the % neutrophil infiltrate/NHP after excluding the lymphocytes and monocytes34 that are shown on the left side y‐axis. DG (334 mg) reduced the colon neutrophil infiltrate score/NHP by 75%. There was no change in the lymphocyte or monocyte infiltrate with either infection or after DG treatment. (d) DG prevented vasculitis as shown on the left side y‐axis. The right side y‐axis shows the % fibrin thrombi score/NHP, which was reduced by 67%. Mean ± SEM with two‐tailed Mann–Whitney test (*P < 0·05). AC, ascending colon; DC, descending colon; TC, transverse colon.
Figure 2.
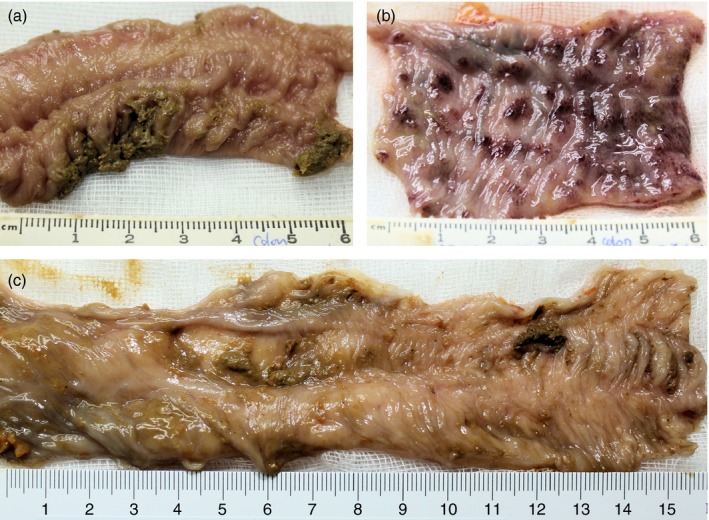
Macroscopic pathology of colon. (a) Mucosal surface of normal rhesus macaque colon. (b) Mucosal surface of Shigella dysenteriae Type‐1‐infected colon at day 6 showing severe haemorrhagic gut wall damage. (c) Mucosal surface of Shigella dysenteriae‐infected colon at day 6 after dendrimer glucosamine (DG) (334 mg) treatment showing minimal damage.
Figure 3.
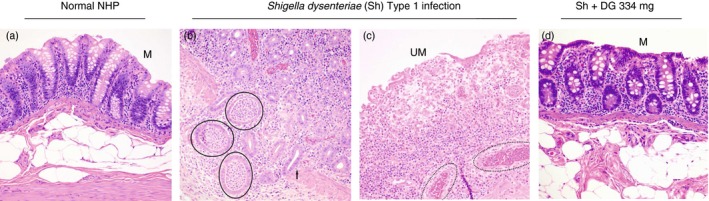
Microscopic histopathology of colon. (a) Normal rhesus macaque colon with mild background lymphoplasmacytic infiltrate in the lamina propria. M = mucosa. (b) Shigella dystenteriae Type‐1‐infected colon. Mucosa has herniated into sub‐epithelial connective tissue and muscularis mucosae (arrow) and is infiltrated by many neutrophils. The herniated glands are abscessed (circles). (c) Shigella‐infected colon. Severe neutrophil infiltration of eroded and ulcerated mucosa (UM) with inflammatory infiltrate extending beyond the lamina propria and into the sub‐epithelial connective tissue and muscularis mucosae. Medium sized arteries in the sub‐mucosa have neutrophils infiltrating and disrupting the full thickness of their vessel walls; this is diagnostic of vasculitis (dotted oval). (d) Shigella‐infected colon + dendrimer glucosamine (DG) 334 mg treatment. There is mild infiltration only of the mucosa (M) by lymphocytes, monocytes and neutrophils. Haematoxylin & eosin (× 200).
Fibrin thrombi and vasculitis represent progressively more severe degrees of blood vessel‐associated tissue injury from infection‐related‐inflammation45, 46 (see Supplementary material, Fig. S1). Strikingly, vasculitis did not occur with DG treatment (Figs 1d and 3) and fibrin thrombi were reduced by 67%; they occurred rarely and with less severity in ileum only.35 In addition, Peyer's patches in the ileum remained intact with no damage to their surface mucosa with DG treatment (Fig. 1d, and see Supplementary material, Fig. S2).
Colon cytokines and tissue mediators
Quantitative mRNA RT‐PCR of ascending, transverse and descending colon (pooled data) showed that DG (334 mg) reduced IL‐8 by 77%, IL‐6 by 77% and IL‐1β by 61%. Interferon‐γ fell by 47·5% but did not reach statistical significance (P = 0·06); TNF‐α (inflammatory), IL‐10 (anti‐inflammatory), FoxP3 and TGF‐β (regulatory T cells23) did not change with infection or DG treatment (Fig. 4, see Supplementary material, Table S1). Interleukin‐4 was usually below the limit of detection47 (data not shown).
Figure 4.
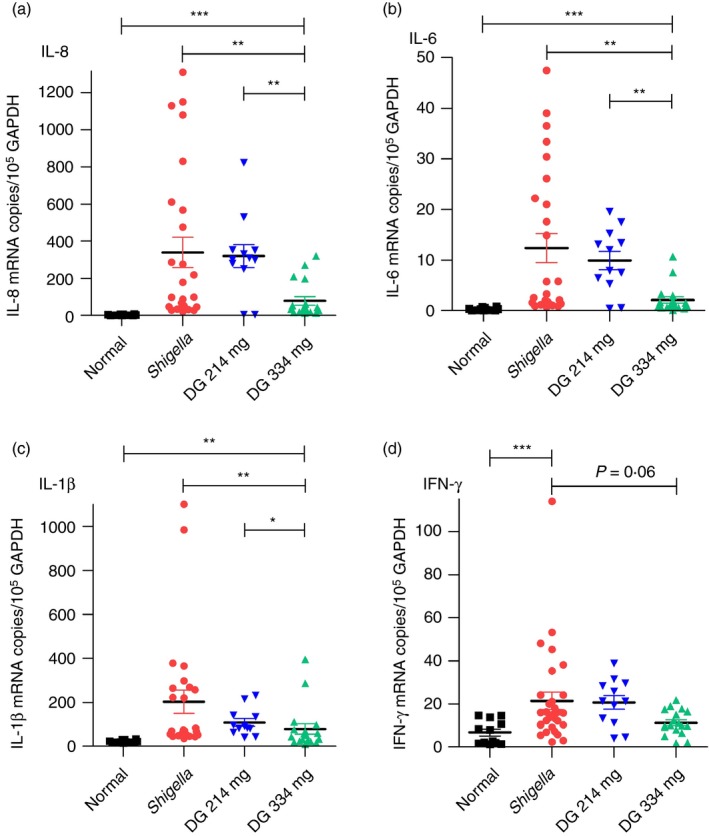
Cytokines in colon. (a–d) Dendrimer glucosamine (DG; 334 mg) reduced interleukin‐8 (IL‐8) by 77%, IL‐6 by 77%, IL‐1β by 61% and interferon‐γ (IFN‐γ) by 48% (P = 0·06) compared with Shigella control on day 6. DG (214 mg) had no effect. Pooled results for the ascending colon, transverse colon and descending colon are shown. Normal (■); Shigella ( ); DG 214 mg (
); DG 214 mg ( ); DG 334 mg (
); DG 334 mg ( ). Scatter plot with mean ± SEM with two‐tailed Mann–Whitney test (*P < 0·05, **P < 0·01, ***P < 0·001).
). Scatter plot with mean ± SEM with two‐tailed Mann–Whitney test (*P < 0·05, **P < 0·01, ***P < 0·001).
Rectum cytokines and tissue mediators
In the rectum, DG (334 mg) reduced IL‐8 by 90%, IL‐1β by 60% and IFN‐γ by 52%. Interleukin‐6 did not change with either infection or DG treatment; TNF‐α (inflammatory), IL‐10 (anti‐inflammatory), FoxP3 and TGF‐β (regulatory T cells23) did not change with either infection or DG treatment (Fig. 5, see Supplementary material, Table S2).
Figure 5.
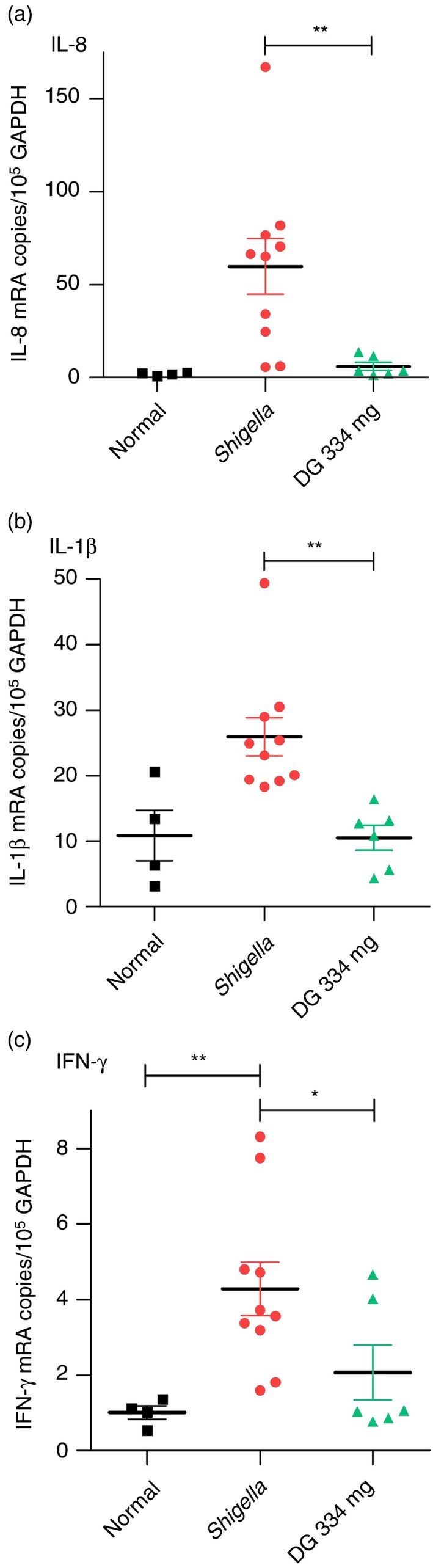
Cytokines in rectum. Dendrimer glucosamine (DG) (334 mg) reduced interleukin‐8 (IL‐8) by 90%, IL‐1β by 60%, and interferon‐γ (IFN‐γ) by 52% when compared with Shigella infection control. DG (214 mg) had no effect. Normal rhesus macaque (■); Shigella infection control ( ); DG 334 mg (
); DG 334 mg ( ). Scatter plot with mean ± SEM with two‐tailed Mann–Whitney test (*P < 0·05, **P < 0·01).
). Scatter plot with mean ± SEM with two‐tailed Mann–Whitney test (*P < 0·05, **P < 0·01).
The diarrhoeal content of stool cytokines peaks on day 5 after infection and was measured for IL‐8, IL‐1β, IL‐6, TNF‐α and IFN‐γ proteins using the FlowMetrix™ System (Luminex Corp., Austin, TX) as described previously.12 Interleukin‐8 and IL‐1β proteins were significantly increased in the diarrhoeal content after infection, and they were also significantly reduced by DG (334 mg) treatment. There was no change in IL‐6, TNF‐α or IFN‐γ proteins in the diarrhoeal content with either infection or after DG treatment.
Colon FACS analysis
The FACS analysis showed that DG (334 mg) did not change either the % CD3+ CD4+ lymphocytes or the % CD3+ CD8+ lymphocytes in colon (Fig. 6a).
Figure 6.
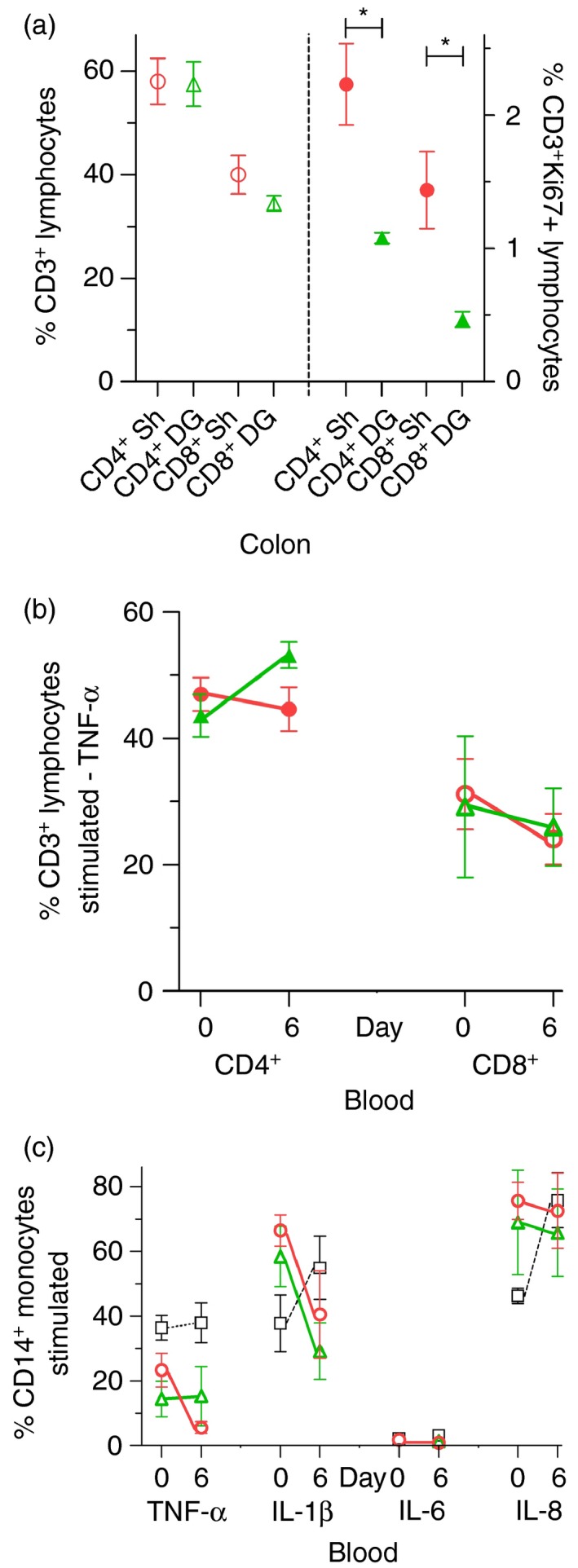
Lymphocyte and monocyte function in colon and blood. (a) Dendrimer glucosamine (DG; 334 mg) did not change the % CD3+
CD4+ lymphocytes or % CD3+
CD8+ lymphocytes in colon but reduced their Ki67 activation on day 6. (b, c) DG (334 mg) had no effect on the % tumour necrosis factor‐α (TNF‐α) ‐producing CD3+
CD4+ lymphocytes and CD3+
CD8+ lymphocytes or on the % TNF‐α, interleukin‐1β (IL‐1β), IL‐6, IL‐8‐producing CD14+ monocytes in blood after in vitro stimulation with PMA/ionomycin/Brefeldin‐A (days 0 and 6). Normal (■/□); Shigella ( ); DG 214 mg (
); DG 214 mg ( ); DG 334 mg(
); DG 334 mg( ). Mean ± SEM with two‐tailed Mann–Whitney test (*P < 0·05).
). Mean ± SEM with two‐tailed Mann–Whitney test (*P < 0·05).
The rhesus macaque is also a reliable human correlate for studying colon intraepithelial lymphocytes; i.e. TCR‐γδ cells.33 They play an important role in: (i) responding to bacteria and intestinal injury, and (ii) promoting epithelial healing and restoration of gut barrier function.48, 49 FACS analysis showed that the % TCR‐γδ cells in colon did not change from normal (9·0 ± 0·77%) with either Shigella infection (11·77 ± 1·41%) or DG (334 mg) treatment (6·75 ± 1·0%); this range is consistent with a report for colon TCR‐γδ cells in normal rhesus macaques.33
Expression of the Ki‐67 nucleoprotein occurs only during the G1/S/G2/M phase of the cell cycle and is strictly associated with cell proliferation.50 Importantly, DG (334 mg) treatment reduced the Ki67 activation of both CD3+ CD4+ lymphocytes and CD3+ CD8+ lymphocytes in colon. For CD3+ CD4+ lymphocytes, Ki67 activation fell by 52% from 2·23 ± 0·3% (Shigella group) to 1·08 ± 0·04% (DG group) (P = 0·03). For CD3+ CD8+ lymphocytes, Ki67 activation fell by 67% from 1·44 ± 0·29% (Shigella group) to 0·47 ± 0·06% (DG group) (P = 0·03) (Fig. 6a).
Blood FACS analysis
Dendrimer glucosamine (334 mg) had no effect on blood lymphocyte TNF‐α production (Fig. 6b) and DG (334 mg) also had no effect on blood monocyte TNF‐α, IL‐1β, IL‐6, IL‐8 production after in vitro stimulation with PMA/ionomycin/Brefeldin‐A (Fig. 6c). DG (214 mg) had no effect on any of the above (data not shown).
Mesenteric lymph node cytokines and tissue mediators
Gut mesenteric lymph nodes in NHPs are very similar to those in humans.33 Neither infection nor DG altered cytokines and tissue mediators in gut mesenteric lymph nodes. This confirmed: (i) the colon and rectum localization of the infection‐related cytokine storm in NHPs; and (ii) the gut wall limited activity of DG (see Supplementary material, Table S3). DG (214 mg) had no effect on any chemokine/cytokine/tissue mediator at any site (data not shown).
Mesenteric lymph node FACS analysis
Neither infection nor DG (i.e. 214 and 334 mg dosing) changed the % CD3+ CD4+ lymphocytes or the % CD3+ CD8+ lymphocytes in lymph nodes (Fig. 7a). After in vitro stimulation with PMA/ionomycin/Brefeldin‐A, neither Shigella infection nor DG treatment changed the % TNF‐α producing CD3+ CD4+ lymphocytes or the CD3+ CD8+ lymphocytes in lymph nodes (Fig. 7b).
Figure 7.
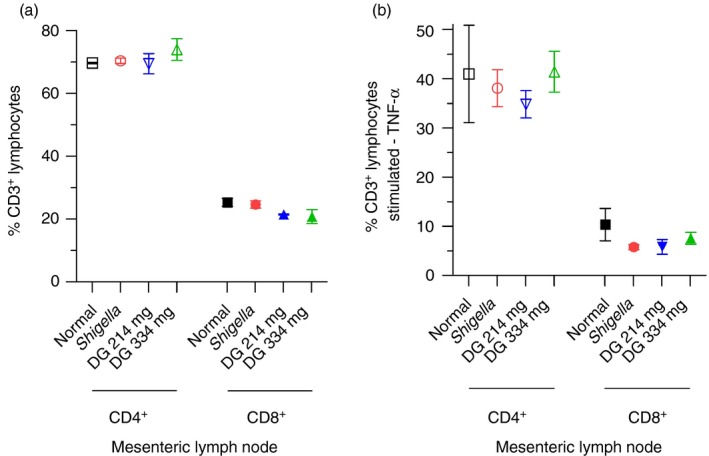
Gut mesenteric lymph nodes. (a) FACS analysis of CD3+
CD4+ lymphocytes and CD3+
CD8+ lymphocytes in gut mesenteric lymph nodes on day 6. Neither infection nor dendrimer glucosamine (DG) changed the % CD3+
CD4+ lymphocytes or the % CD3+
CD8+ lymphocytes in lymph nodes. (b) FACS analysis of CD3+
CD4+ lymphocytes and CD3+
CD8+ lymphocytes in gut mesenteric lymph nodes after in vitro stimulation with PMA/ionomycin/Brefeldin‐A. Neither infection nor DG treatment changed the % tumour necrosis factor‐α (TNF‐α) ‐producing CD3+
CD4+ lymphocytes or CD3+
CD8+ lymphocytes in lymph nodes. Normal rhesus macaque (□/■); Shigella infection control ( ); DG 214 mg (
); DG 214 mg ( ); DG 334 mg (
); DG 334 mg ( ). Mean ± SEM with two‐tailed Mann–Whitney test (*P < 0·05).
). Mean ± SEM with two‐tailed Mann–Whitney test (*P < 0·05).
Discussion
Here we show that in the most severe form of Gram‐negative bacterial diarrhoea and colitis, the oral TLR4 antagonist DG protected the rhesus macaque colon and rectum from pro‐inflammatory cytokine‐induced and neutrophil‐mediated vasculitis and gut wall necrosis. Colon CD4+ and CD8+ lymphocyte activation was also reduced by DG. Importantly, the systemic innate immune cytokine response in both gut lymph nodes and blood remained intact and undisturbed.
By performing stool cultures twice daily after the administration of 2 × 109 CFUs of S. dysenteriae Type 1 1617 into the stomach, we were able to show that the time to the first stool positive for Shigella was 1·6 ± 0·2 days for all three groups studied. This infectious diarrhoea was then allowed to progress for another 4 days. The only medical intervention was to ensure adequate hydration with subcutaneous infusion of water, sodium and potassium if the NHPs stopped drinking; anorexia is common in severe sepsis. This study is likely to be the closest to a study in humans of an oral TLR4 immunomodulator in severe sepsis that could be performed because a human clinical trials ethics committee would not allow the exclusion of antibiotics.
During the first 2 days of any severe bacterial infection, the immediate innate immune response tries to eradicate bacteria from the gut at the cost of mucosal destruction. All pathogenic Gram‐negative bacteria stimulate local tissue macrophages to produce IL‐6 and IL‐1β via TLR4;1, 6 the gut‐associated lymphoid tissue contains the largest body pool of macrophages.51 This leads to the recruitment of IL‐8‐ and IL‐1β‐producing blood monocytes into the gut by day 2–3, and of IL‐8‐producing blood neutrophils by day 3 after infection.52 Monocytes and neutrophils have their own independent programmes for recruitment into tissues.53
Our new finding of a large reduction of IL‐8, IL‐6, IL‐1β and IFN‐γ in the colon and rectum of NHPs (Figs 4 and 5) is consistent with DG blocking the cell surface TLR4–MYD88 early cytokine pathway of the TLR4‐MD‐2‐LPS receptor complex while sparing the endosomal TLR4‐TRIF/TRAM late cytokine and IFN‐β/CD86 pathway.16, 17, 18, 19, 20, 21 Maintaining TRIF signalling ensures an adequate gut pro‐inflammatory cytokine response for recruiting the neutrophils required to prevent the spread of pathogenic bacteria into tissues and blood. It is important to remember that activation of the TRIF pathway of TLR4 results in a mild rather than a severe pro‐inflammatory cytokine response.19 TRIF signalling also ensures that mucosal antimicrobial peptide production (i.e. natural antibiotics) continues with these peptides acting to control local bacterial growth and replication.23, 54, 55 Furthermore, stimulation of TRIF leads to sequential expression of IFN‐β and IL‐27p28 in macrophages (in response to gut commensal bacteria) with signal transducer and activator of transcription 1 activation also ensuring maintenance of gut wall T helper type 17 cells.56
Vasculitis reflects the severity of neutrophil and Shiga toxin‐induced injury to the colon after infection, and is associated with high patient mortality.9, 11, 14, 15, 47 The relative contribution of neutrophil lysosomal enzymes versus Shiga toxin to this tissue damage has been difficult to define precisely. However, two published observations are noteworthy: (i) Fulminant and lethal bloody diarrhoea can occur even when a toxin‐negative mutant of S. dysenteriae Type 1 causes infection;14 and (ii) in a rat model of mercuric chloride induced vasculitis of the colon, neutrophils were necessary for induction of vasculitis with the degree of vasculitis correlating with the number of neutrophils present.57 In our study, the absence of tissue vasculitis combined with a 67% reduction in tissue fibrin thrombi with DG treatment (Figs 1d and 3) indicated a substantial reduction in infection‐related blood vessel damage, and correlated with a 75% reduction in colon neutrophil infiltration. This absence of tissue vasculitis with DG treatment was a surprising and unexpected result. Taken together, our observations suggest that the pro‐inflammatory cytokine storm is primarily responsible for initiating the neutrophil‐mediated pathology that leads to tissue vasculitis.
DG treatment did not alter the % CD3+ CD4+ lymphocytes, CD3+ CD8+ lymphocytes or TCR‐γδ cells in colon (Fig. 7a). Ki‐67+ proliferation of lymphocytes in the rectum of patients with shigellosis has been described,44, 50 and was also seen in the colon of our infected NHPs. Notably, DG treatment reduced Ki67 activation of CD4+ and CD8+ lymphocytes in colon by 52 and 67%, respectively (Fig. 7a). These results show that infection‐induced and lymphocyte‐associated inflammation of the colon was reduced by DG treatment.
The broad implication of our new findings with an oral TLR4 antagonist given once daily for just 5 days is to define an alternative strategy for protecting the gut epithelial barrier from bacteria‐induced and cytokine‐ and neutrophil‐mediated tissue damage. Such gut wall barrier breakdown is increasingly recognized as a major driving force in precipitating systemic sepsis‐induced multi‐organ failure.58 Our results further support this growing recognition and also show that, in NHPs, IL‐8 is as important as IL‐6 in destroying the epithelial gut wall's integrity, promoting bacterial tissue invasion, and leading to severity of clinical disease seen. Our observations are also consistent with the dramatic increase in IL‐8 that occurs in human shigellosis and point to this chemokine being the major orchestrator of gut mucosal inflammation in severe infectious diarrhoeal diseases;59 this is consistent with the neutrophil recruiting and activating role of IL‐8 in acutely infected tissues.
Taken together, this leads us to propose that non‐absorbable oral TLR4 antagonists could be started at the same time as antibiotics in patients with symptoms and signs of infection to protect and preserve the gut wall barrier. The importance of maintaining the competence of a crucial body barrier (i.e. the gut wall) is that it minimizes the likelihood of bacterial tissue invasion. Strategies for orally based delivery of drugs to the colon are already well established using hard hypromellose capsules with pH‐sensitive methacrylic acid copolymer coatings (Eudragit®).60
A direct consequence of the approach proposed would be significantly reduced systemic bacterial disease severity. This therapeutic approach should also mean much shorter courses of antibiotics; e.g. 3 days versus 14 days. This would impact immediately on the global antimicrobial crisis, without further increasing bacterial resistance.
Disclosures
The authors declare no conflict of interest.
Supporting information
Figure S1. Shigella dysenteriae Type 1 infected rhesus macaque – vasculitis (day 6). (a) Mural vasculitis predominated by neutrophils admixed with some lymphocytes and eosinophils (haematoxylin & eosin; H&E, × 400). (b) Vasculitis in the lamina propria of a villus in the ileum that has inflammatory cells invading and expanding the lamina adventitia and media of the blood vessel wall (H&E, × 400). (c) Jejunum showed occasional evidence of fibrin thrombi in the lamina propria of villi. There is limited inflammation surrounding the fibrin thrombi (H&E, × 400).
Figure S2. Shigella dysenteriae Type 1 infected rhesus macaque + DG 334 mg – jejunum and ileum (day 6). (a) Jejunum. There is no damage to the mucosa. The background lymphocyte infiltrate is within normal limits for rhesus macaque (haematoxylin & eosin; H&E, × 100). (b) Ileum. An intact Peyer's patch in the ileum with an intact mucosa (H&E, × 200).
Table S1. Colon mRNA summary data.
Table S2. Rectum mRNA summary data.
Table S3. Mesenteric lymph node mRNA summary data.
Acknowledgements
SS, DI, IT and CJM conceived and designed the study. RI was the vet and EL was the pathologist. DI, NR, PK, PN, SG, and KY performed the animal study and tissue analysis. The manuscript was written by SS, DI, EL, IT, BES and CJM with input from all authors. We are grateful for the technical support provided by Kesara Chumpolkulwong and Alongkorn Hanrujirakomjorn for veterinary procedures, Amnat Andang, Rachata Jecksaeng, Mana Saitasao and Siwakorn Sirisrisopa for husbandry, and Paphavee Lertsethtakarn, Sathit Pichyangkul, Ajchar Aksomboon, Nuanpan Khemnu, Kaewkanya Nakjarung and Sasikorn Silapong for assays. This study was funded by NIAID under Inter‐Agency Agreement Y1‐AI‐4906‐03 and US Army Medical Research & Materiel Command and Imperial Biomedical Research Centre and Williams Trust grants to CJM, BES and SS.
References
- 1. Tsolis RM, Young GM, Solnick JV, Bäumler AJ. From bench to bedside: stealth of enteroinvasive pathogens. Nat Rev Microbiol 2008; 6:883–92. [DOI] [PubMed] [Google Scholar]
- 2. LaRock DL, Chaudhary A, Miller SI. Salmonellae interactions with host processes. Nat Rev Microbiol 2015; 13:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keestra‐Gounder AM, Tsolis RM, Bäumler AJ. Now you see me, now you don't: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol 2015; 13:206–16. [DOI] [PubMed] [Google Scholar]
- 4. Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A et al Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS–NO‐nitrite signalling. Proc Natl Acad Sci USA 2013; 110:9451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benkoe TM, Mechtler TP, Weninger M, Pones M, Rebhandl W, Kasper DC. Serum levels of IL‐8 and gut‐associated biomarkers in diagnosing necrotizing enterocolitis in preterm infants. J Pediatr Surg 2014; 49:1446–51. [DOI] [PubMed] [Google Scholar]
- 6. Khan MA, Ma C, Knodler LA, Valdez Y, Rosenberger CM, Deng W et al TLR4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect Immun 2006; 74:2522–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fournier BM, Oarkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol 2012; 5:354–66. [DOI] [PubMed] [Google Scholar]
- 8. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W et al Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 2013; 110:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raqib R, Lindberg AA, Wretlind B, Bardhan PK, Andersson U, Andersson J. Persistence of local cytokine production in Shigellosis in acute and convalescent stages. Infect Immun 1995; 63:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schnupf P, Sansonetti PJ. Quantitative RT‐PCR profiling of the rabbit immune response: assessment of acute Shigella flexneri infection. PLoS ONE 2012; 6:e36446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harendra de Silva DG, Mendis LN, Sheron N, Alexander GJ, Candy DC, Chart H et al Concentrations of IL‐6 and TNF in serum and stools of children with Shigella dysenteriae 1 infection. Gut 1993; 34:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Islam D, Ruamsap N, Khantapura P, Aksomboon A, Srijan A, Wongstitwilairoong B et al Evaluation of an intragastric challenge model for Shigella dysenteriae 1 in rhesus monkeys (Macaca mulatta) for the pre‐clinical assessment of Shigella vaccine formulations. APMIS 2012; 122:463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gregory M, Kaminski RW, Lugo‐Roman LA, Galvez Carrillo H, Tilley DH, Baldeviano C et al Development of an Aotus nancymaae model for Shigella vaccine immunogenicity and efficacy studies. Infect Immun 2014; 82:2027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fontaine A, Arondel J, Sansonetti PJ. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox‐mutant of Shigella dysenteriae Type 1. Infect Immun 1988; 56:3099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA et al Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci USA 2006; 103:9178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H et al Role of adaptor TRIF in the MyD88‐independent TLR signalling pathway. Science 2003; 301:640–3. [DOI] [PubMed] [Google Scholar]
- 17. Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of TLR4 to the induction of IFN‐β . Nat Immunol 2008; 9:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watts C. Location, location, location: identifying the neighborhoods of LPS signaling. Nat Immunol 2008; 9:343–5. [DOI] [PubMed] [Google Scholar]
- 19. Needham BD, Trent MS. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol 2013; 11:467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaunak S, Thomas S, Gianasi E, Godwin A, Jones E, Teo I et al Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat Biotechnol 2004; 22:977–84. [DOI] [PubMed] [Google Scholar]
- 21. Shaunak S. Dendrimers drugs for infection & inflammation. Biochem Biophys Res Commun 2015; doi:10.1016/j.bbrc.2015.07.033. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22. Barata TS, Teo I, Brocchini S, Zloh M, Shaunak S. Partially glycosylated dendrimers block MD‐2 and prevent TLR4‐MD‐2‐LPS complex mediated cytokine responses. PLoS Comput Biol 2011; 7:e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teo I, Toms SM, Marteyn B, Barata TS, Simpson P, Johnston KA et al Preventing acute gut wall damage in infectious diarrhoeas with glycosylated dendrimers. EMBO Mol Med 2012; 4:866–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelleher D. Dendrimers branch out to support mucosal integrity. EMBO Mol Med 2012; 4:860–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asquith M, Haberthur K, Brown M, Engelmann F, Murphy A, Al‐Mahdi Z et al Age‐dependent changes in innate immune phenotype and function in rhesus macaques (Macaca mulatta). Pathobiol Aging Age Relat Dis 2012; 2:18052. doi: 10.3402/pba.v2i0.18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vongsawan AA, Kapatral V, Vaisvil B, Burd H, Serichantalergs O, Venkatesan MM et al The genome of Shigella dysenteriae strain Sd1617 comparison to representative strains in evaluating pathogenesis. FEMS Microbiol Lett 2015; 362:fnv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Villinger F, Brar SS, Mayne A, Chikkala N, Ansari AA. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J Immunol 1995; 155:3946–54. [PubMed] [Google Scholar]
- 28. Abel K, Alegria‐Hartman MJ, Zanotto K, McChesney MB, Marthas ML, Miller CJ. Anatomic site and immune function correlate with relative cytokine mRNA expression levels in lymphoid tissues of normal rhesus macaques. Cytokine 2001; 16:191–204. [DOI] [PubMed] [Google Scholar]
- 29. Jovanovic M, Rooney MS, Mertins P, Przybylski D, Chevrier N, Satija R et al Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens. Science 2015; 347:1259038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verhoeven D, Sankaran S, Silvey M, Dandekar S. Antiviral therapy during primary SIV infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. J Virol 2008; 82:4016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benveniste O, Vaslin B, Le Grand R, Cheret A, Matheux F, Theodoro F et al Comparative IL‐2/IFN‐γ and IL‐4/IL‐10 responses during acute infection of macaques inoculated with attenuated nef‐truncated or pathogenic SICmac251 virus. Proc Natl Acad Sci USA 1996; 93:3658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gansuvd B, Asiedu CK, Goodwin J, Jargal U, Deckard LA, Andrades P et al Expansion of CD4+CD25+ suppressive regulatory T cells from rhesus macaque peripheral blood by FN18/antihuman CD28‐coated Dynal beads. Hum Immunol 2007; 68:478–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veazey RS, Rosenzweig M, Shvetz DE, Pauley DR, DeMaria M, Chalifoux LV et al Characterization of gut‐associated lymphoid tissue (GALT) of normal rhesus macaques. Clin Immunol Immunopathol 1997; 82:230–42. [DOI] [PubMed] [Google Scholar]
- 34. Sestak K, Merritt CK, Borda J, Saylor E, Schwamberger SR, Cogswell F et al Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect Immun 2003; 71:4079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rout WR, Formal SB, Giannella RA, Dammin GJ. Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterology 1975; 68:270–8. [PubMed] [Google Scholar]
- 36. Autissier P, Soulas C, Burdo TH, Williams KC. Immunophenotyping of lymphocyte, monocyte and dendritic cell subsets in normal rhesus macaques by 12‐color flow cytometry. J Immunol Meth. 2010; 360:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kotloff KL, Herrington DA, Hale TL, Newland JW, Van De Verg L, Cogan JP et al Safety, immunogenicity, and efficacy in monkeys and humans of invasive E. coli K‐12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infect Immun 1992; 60:2218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hukkanen RR, Liggitt HD, Murnane RD, Frevert CW. Systemic inflammatory response syndrome in non‐human primates culminating in multiple organ failure, acute lung injury, and disseminated intravascular coagulation. Toxicologic Pathol 2009; 37:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cary M, Kosanke S, White G. Spontaneous Waterhouse–Friderichsen syndrome in a gang‐housed baboon. J Med Primatol 2001; 30:185–7. [DOI] [PubMed] [Google Scholar]
- 40. Bennish ML, Salam MA, Wahed MA. Enteric protein loss during Shigellosis. Am J Gastroenterol 1993; 88:53–7. [PubMed] [Google Scholar]
- 41. François M, Le Cabec V, Dupont M‐A, Sansonetti PJ, Maridonneau‐Parini I. Induction of necrosis in human neutrophils by Shigella flexneri requires Type III secretion, IpaB and IpaC invasins, and actin polymerization. Infect Immun 2000; 68:1289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hathaway LJ, Griffin GE, Sansonetti PJ, Edgeworth JD. Human monocytes kill Shigella flexneri but then die by apoptosis associated with suppression of proinflammatory cytokine production. Infect Immun 2002; 70:3833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sabroe I, Dower SK, Whyte MK. The role of Toll‐like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis 2005; 41:S421–6. [DOI] [PubMed] [Google Scholar]
- 44. Raqib R, Moly PK, Sarker P, Qadri F, Alam NH, Mathan M et al Persistence of mucosal mast cells and eosinophils in Shigella‐infected children. Infect Immun 2003; 71:2684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology 2010; 56:3–23. [DOI] [PubMed] [Google Scholar]
- 46. Heeringa P, Foucher P, Klok PA, Huitema MG, Tervaert JW, Weening JJ et al Systemic injection of products of activated neutrophils and H2O2 in myeloperoxidase‐immunized rats leads to necrotizing vasculitis in the lungs and gut. Am J Pathol 1997; 151:131–40. [PMC free article] [PubMed] [Google Scholar]
- 47. Samandari T, Kotloff KL, Losonsky GA, Picking WD, Sansonetti PJ, Levine MM et al Production of IFN‐γ and IL‐10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin‐deleted Shigella dysenteriae type 1 strain. J Immunol 2000; 164:2221–32. [DOI] [PubMed] [Google Scholar]
- 48. Rakasz E, MacDougall AV, Zayas MT, Helgelund JL, Ruckward TJ, Hatfield G et al γδ T cell receptor repertoire in blood and colonic mucosa of rhesus macaques. J Med Primatol 2000; 29:387–96. [DOI] [PubMed] [Google Scholar]
- 49. Smith PM, Garrett WS. The gut microbiota and mucosal T cells. Front Microbiol 2011; 2:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Islam D, Veress B, Bardhan PK, Lindberg AA, Christensson B. In situ characterization of inflammatory responses in the rectal mucosae of patients with shigellosis. Infect Immun 1997; 65:739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bain CC, Bravo‐Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S et al Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 2014; 15:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kucharzik T, Hudson JT III, Lügering A, Abbas JA, Bettini M, Lake JG et al Acute induction of human IL‐8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut 2005; 54:1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dal‐Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B et al A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med 2015; 212:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B et al Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med 2001; 7:180–5. [DOI] [PubMed] [Google Scholar]
- 55. Stockinger S, Duerr CU, Fulde M, Dolowschiak T, Pott J, Yang I et al TRIF signaling drives homeostatic intestinal epithelial antimicrobial peptide expression. J Immunol 2014; 193:4223–34. [DOI] [PubMed] [Google Scholar]
- 56. Kanagavelu S, Flores C, Termini JM, Riveron R, Romero L, Chung K et al TIR‐domain‐containing adapter‐inducing IFN‐β (TRIF) regulates Th17‐mediated intestinal immunopathology in colitis. Mucosal Immunol 2015; 8:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qasim FJ, Mathieson PW, Sendo F, Thiru S, Oliveira DB. Role of neutrophils in the pathogenesis of experimental vasculitis. Am J Pathol 1996; 149:81–9. [PMC free article] [PubMed] [Google Scholar]
- 58. Schulz K, Sommer O, Jargon D, Utzolino S, Clement HW, Strate T et al Cytokine and radical inhibition in septic intestinal barrier failure. J Surg Res 2015; 193:831–40. [DOI] [PubMed] [Google Scholar]
- 59. Pédron T, Thibault C, Sansonetti PJ. The invasive phenotype of Shigella flexneri directs a distinct gene expression pattern in the human intestinal epithelial cell line Caco‐2. J Biol Chem 2003; 278:33878–86. [DOI] [PubMed] [Google Scholar]
- 60. Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano‐delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine 2015; 11:1117–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Shigella dysenteriae Type 1 infected rhesus macaque – vasculitis (day 6). (a) Mural vasculitis predominated by neutrophils admixed with some lymphocytes and eosinophils (haematoxylin & eosin; H&E, × 400). (b) Vasculitis in the lamina propria of a villus in the ileum that has inflammatory cells invading and expanding the lamina adventitia and media of the blood vessel wall (H&E, × 400). (c) Jejunum showed occasional evidence of fibrin thrombi in the lamina propria of villi. There is limited inflammation surrounding the fibrin thrombi (H&E, × 400).
Figure S2. Shigella dysenteriae Type 1 infected rhesus macaque + DG 334 mg – jejunum and ileum (day 6). (a) Jejunum. There is no damage to the mucosa. The background lymphocyte infiltrate is within normal limits for rhesus macaque (haematoxylin & eosin; H&E, × 100). (b) Ileum. An intact Peyer's patch in the ileum with an intact mucosa (H&E, × 200).
Table S1. Colon mRNA summary data.
Table S2. Rectum mRNA summary data.
Table S3. Mesenteric lymph node mRNA summary data.


