Summary
Young‐onset calorie restriction (CR) in rodents decreases serum IGF‐1 concentration and increases serum corticosterone levels, which have been hypothesized to play major roles in mediating its anticancer and anti‐aging effects. However, little is known on the effects of CR on the IGF‐1 system and cortisol in humans. To test the sustained effects of CR on these key hormonal adaptations, we performed a multicenter randomized trial of a 2‐year 25% CR intervention in 218 nonobese (body mass index between 22 and 27.8 kg m−2) young and middle‐aged (20–50 years age range) men and women. Average CR during the first 6 months was 19.5 ± 0.8% and 9.1 ± 0.7% over the next 18 months of the study. Weight loss averaged 7.6 ± 0.3 kg over the 2‐years period of which 71% was fat mass loss (P < 0.0001). Average CR during the CR caused a significant 21% increase in serum IGFBP‐1 and a 42% reduction in IGF‐1:IGFBP‐1 ratio at 2 years (P < 0.008), but did not change IGF‐1 and IGF‐1:IGFBP‐3 ratio levels. Serum cortisol concentrations were slightly but significantly increased by CR at 1 year only (P = 0.003). Calorie restriction had no effect on serum concentrations of PDGF‐AB and TGFβ‐1. We conclude, on the basis of the present and previous findings, that, in contrast to rodents, humans do not respond to CR with a decrease in serum IGF‐1 concentration or with a sustained and biological relevant increase in serum cortisol. However, long‐term CR in humans significantly and persistently increases serum IGFBP‐1 concentration.
Keywords: calorie restriction, cancer, cortisol, IGF‐1, IGFBP‐1, weight loss
Introduction
Data from experimental and epidemiological studies indicate that insulin‐like growth factor (IGF)‐1 and its binding proteins play a role in the biology of aging and in the pathogenesis of several common cancers (Yu & Rohan, 2000; Renehan et al., 2004; Yakar et al., 2005; Fontana et al., 2010; Kopchick et al., 2014). Patients with acromegaly, who have high growth hormone and IGF‐1 levels, experience a 2‐fold increased risk of gastrointestinal cancers (Renehan et al., 2003), whereas patients with congenital deficiencies in IGF‐1 seem to be protected against the development of cancer (Shevah & Laron, 2007; Guevara‐Aguirre et al., 2011). Data from several genetic animal models of longevity have shown that reduced function mutations in the IGF‐1 signaling pathway have low circulating IGF‐1 levels, reduced cancer incidence, and increased maximal lifespan (Fontana et al., 2010). Moreover, in a study of 31 genetically diverse inbred mouse strains, the median lifespan was inversely correlated with plasma IGF‐1 levels (Yuan et al., 2009). IGF‐1 is a potent mitogenic growth factor, which promotes cell proliferation and differentiation, and inhibits apoptosis (Yakar et al., 2005). The inhibition of the IGF‐1 pathway causes several cellular and metabolic adaptations, including downregulation of growth pathways, upregulation of autophagic and apoptotic pathways, increased resistance to multiple toxic agents, and increased genome stability (Fontana et al., 2010; Bartke et al., 2013).
Calorie restriction (CR) without malnutrition is one of the most powerful interventions to slow aging and prevent cancer in laboratory strains of rodents (Albanes, 1987; Fontana et al., 2010; Longo & Fontana, 2010). Adult‐onset moderate CR also reduces cancer incidence by more than 50% in Rhesus monkeys (Colman et al., 2009; Mattison et al., 2012). It has been hypothesized that the powerful inhibitory effect of CR on spontaneous, chemically induced and radiation‐induced tumors can be mediated, at least in part, by a reduction in IGF‐1 levels and an increase in corticosteroid levels (Longo & Fontana, 2010). In rodents, CR decreases serum insulin‐like growth factor‐1 (IGF‐1) concentration by 20–40% and increases serum corticosterone levels by 30–50% (Breese et al., 1991; Sabatino et al., 1991; Dunn et al., 1997). Both adrenalectomy and IGF‐I supplementation abrogate the protective effect of CR on neoplastic progression (Pashko & Schwartz, 1992, 1996; Dunn et al., 1997; Stewart et al., 2005). Data from two small short‐term (6 and 12 months) randomized clinical trials and a cross‐sectional observational study have shown that CR does not increase serum cortisol or reduce serum IGF‐1 and IGF‐1:IGFBP‐3 ratio levels, unless protein intake is also reduced (Weiss et al., 2006; Fontana et al., 2008; Redman et al., 2010; Tam et al., 2014). However, there are currently no randomized controlled trials (RCT) in young–middle age lean or slightly overweight men and women, evaluating the long‐term effects of CR on serum IGF‐1, IGFBPs, and cortisol concentrations.
One of the purposes of this 2‐year multicenter randomized controlled trial (CALERIE) was to evaluate the effects of a 25% reduction in energy intake in a large number of nonobese young and middle‐aged men and women on the IGF‐1 axis, and other growth and hormonal factors modulated by CR, which have been implicated in the biology of aging and in the pathophysiology of cancer, such as PDGF‐AB, TGF‐β‐1, and cortisol.
Results
Of the more than 10 000 men and women assessed for eligibility, the screening procedures excluded 45% of them for their age or body mass index (BMI), 14% for health or medication reasons, and 30% refused to participate due to concerns about their ability to adhere to the protocol, and personal or study‐related issues (Ravussin et al., 2015). Of the 238 participants who began baseline assessments, 220 were randomized, 218 started the assigned intervention, and 82% of CR and 95% of ad libitum (AL), respectively, completed the study (Fig. 1). Table 1 presents sex, age, race, and BMI data for the two groups. Analysis revealed no significant differences between groups at baseline for these variables.
Figure 1.
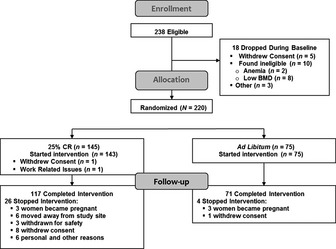
CONSORT diagram.
Table 1.
Demographic, anthropometric, and clinical characteristics at baseline for the 218 participants who started the 2‐year intervention
| Ad libitum (n = 75) | Calorie restriction (n = 143) | |
|---|---|---|
| Race | ||
| White | 57 (76%) | 111 (77.6%) |
| African American | 11 (14.7%) | 15 (10.5%) |
| Other | 7 (9.3%) | 17 (11.9%) |
| Age (years) | 37.9 (6.94) | 38.0 (7.34) |
| Height (m) | 168.4 (8.31) | 168.9 (8.60) |
| Baseline weight (kg) | 71.5 (8.65) | 72.0 (9.49) |
| Baseline BMI (kg m−2) | 25.1 (1.64) | 25.2 (1.78) |
| Body fat (%) | 33.6 (6.57) | 32.9 (6.07) |
| FFM (kg) | 47.6 (8.61) | 48.5 (9.21) |
| Energy and macronutrient intake | ||
| Energy intake (kcal day−1) | 2045.3 (480.66) | 2126.3 (558.60) |
| Protein (g kg−1 day−1) | 1.2 (0.04) | 1.2 (0.02) |
| Protein, % of energy | 17.2 (3.48) | 16.6 (3.04) |
| Fat, % of energy | 34.7 (5.12) | 33.5 (4.93) |
| Carbohydrates, % of energy | 45.1 (6.33) | 46.8 (6.48) |
| Laboratory values | ||
| IGF‐1 (ng mL−1) | 183.1 (49.40) | 175.5 (42.66) |
| IGFBP‐1 (pg mL−1) | 4477 (5033.7) | 5459 (6252.0) |
| IGFBP‐3 (pg mL−1) | 2529 (502.7) | 2459 (399.8) |
| IGF‐1/IGFBP‐3 ratio | 0.07 (0.025) | 0.07 (0.020) |
| IGF‐1/IGFBP‐1 ratio | 0.10 (0.105) | 0.08 (0.092) |
| PDGF‐AB (ng mL−1) | 20.0 (6.01) | 18.1 (6.97) |
| TGF‐β1 (ng mL−1) | 30.6 (8.02) | 28.9 (9.72) |
| Cortisol (μg dL−1) | 11.3 (5.91) | 11.2 (4.92) |
AL, ad libitum control group; CR, 25% calorie restriction group; FFM, fat‐free mass; FM, fat mass.
Values represent means (SD).
Intervention adherence, body weight, and composition
Detailed information regarding the observed adherence to the intervention has been published previously (Ravussin et al., 2015). At baseline, there was no difference in average energy intake (assessed as TDEE during weight stability) between the CR [2467 (34) kcal day−1] and AL [2390 (45) kcal day−1] groups (P = 0.15). In the CR group, energy intake was reduced by 19.5 (0.8)% (480 kcal day−1) during the first 6 months, and by an average of 9.1 (0.7)% (234 kcal day−1) below baseline for the remaining 18 months of the study (P < 0.0001 vs. AL control group), while in the control group average daily energy intake was unchanged (Table 2). Weight loss averaged 8.3 (0.3) kg (11.5%) at 1 year and 7.6 (0.3) kg (10.4%) at 2 years in the CR group (P < 0.001), but did not change significantly in the AL group (Table 2). Body fat measured by dual‐energy X‐ray absorptiometry (DXA) decreased by 6.1 (0.2) kg at 1 year and 5.3 (0.3) kg at 2 years in the CR group (P < 0.001), but did not change in the AL group. Fat loss accounted for ~71% of the weight loss (Table 2).
Table 2.
Change from baseline in body composition at 12 and 24 months in AL and calorie restriction (CR) groups
| Outcome | AL | CR | Between‐group P‐valueb | ||
|---|---|---|---|---|---|
| Mean (SE)a | Within‐group P‐valueb | Mean (SE)a | Within‐group P‐valueb | ||
| Clinical weight (kg) | |||||
| Baseline | 71.5 (1.0) | 72.0 (0.8) | 0.978 | ||
| Δ Month 12 | −0.7 (0.4) | 0.105 | −8.4 (0.3) | < 0.001 | < 0.001 |
| Δ Month 24 | 0.1 (0.5) | 1.0 | −7.5 (0.4) | < 0.001 | < 0.001 |
| Body mass index (kg m−2) | |||||
| Baseline | 25.1 (0.2) | 25.2 (0.2) | 0.937 | ||
| Δ Month 12 | −0.2 (0.1) | 0.207 | −2.9 (0.1) | < 0.001 | < 0.001 |
| Δ Month 24 | 0.1 (0.2) | 1.0 | −2.6 (0.1) | < 0.001 | < 0.001 |
| % Body fat | |||||
| Baseline | 33.6 (0.8) | 32.9 (0.5) | 0.336 | ||
| Δ Month 12 | −0.47 (0.3) | 0.254 | −5.5 (0.2) | < 0.001 | < 0.001 |
| Δ Month 24 | 0.13 (0.3) | 1.0 | −4.6 (0.3) | < 0.001 | < 0.001 |
| Fat mass (kg) | |||||
| Baseline | 23.8 (0.6) | 23.5 (0.4) | 0.611 | ||
| Δ Month 12 | −0.34 (0.3) | 0.518 | −6.1 (0.2) | < 0.001 | < 0.001 |
| Δ Month 24 | 0.38 (0.4) | 0.564 | −5.3 (0.3) | < 0.001 | < 0.001 |
| Fat‐free mass (kg) | |||||
| Baseline | 47.6 (1.0) | 48.5 (0.8) | 0.475 | ||
| Δ Month 12 | −0.3 (0.2) | 0.131 | −2.2 (0.1) | < 0.001 | < 0.001 |
| Δ Month 24 | −0.2 (0.2) | 0.837 | −2.2 (0.2) | < 0.001 | < 0.001 |
Baseline values are the observed mean (SE); change scores are the least‐squares adjusted means (SE) from the ITT repeated measures analysis.
Within‐group P‐value tests for a significant change from baseline to the follow‐up time point in that group; between‐group P‐value tests for a significant between‐group difference in the change score at the time point. All P‐values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text).
Self‐reported energy and nutrient intake
Seven‐day food records showed that the CR group significantly restricted their energy intake [−279 (29) kcal day−1 at 1 year and −216 (33) kcal d−1 at 2 years], while the AL [−83 (38) kcal day−1 at 1 year and −121 (43) kcal day−1 at 2 years] group maintained its intake (Table 3). Accordingly, intake of macronutrients such as fat also decreased in the CR compared with the AL group, but protein intake increased significantly (Table 3).
Table 3.
Change from baseline in dietary energy intake and macronutrient composition at 12 and 24 months in AL and calorie restriction (CR) groups
| Outcome | AL | CR | Between‐group P‐valueb | ||
|---|---|---|---|---|---|
| Mean (SE)a | Within‐group P‐valueb | Mean (SE)a | Within‐group P‐valueb | ||
| Energy intake (kcal day−1) | |||||
| Baseline | 2045.3 (55.5) | 2126.3 (46.7) | 0.463 | ||
| Δ Month 12 | −83.0 (38.6) | 0.065 | −279.5 (29.3) | < 0.001 | < 0.001 |
| Δ Month 24 | −121.1 (42.7) | 0.010 | −216.3 (33.1) | < 0.001 | 0.073 |
| Protein intake (g kg−1) | |||||
| Baseline | 1.2 (0.04) | 1.2 (0.02) | 0.951 | ||
| Δ Month 12 | −0.002 (0.03) | 1.0 | 0.11 (0.03) | < 0.001 | 0.007 |
| Δ Month 24 | −0.09 (0.04) | 0.055 | 0.08 (0.03) | 0.012 | 0.001 |
| % Calories from protein | |||||
| Baseline | 17.2 (0.4) | 16.6 (0.3) | 0.252 | ||
| Δ Month 12 | 0.8 (0.4) | 0.123 | 1.8 (0.3) | < 0.001 | 0.047 |
| Δ Month 24 | 0.1 (0.4) | 1.0 | 1.1 (0.3) | 0.003 | 0.068 |
| % Calories from fat | |||||
| Baseline | 34.7 (0.6) | 33.5 (0.4) | 0.034 | ||
| Δ Month 12 | 0.2 (0.6) | 1.0 | −4.8 (0.4) | < 0.001 | < 0.001 |
| Δ Month 24 | 0.5 (0.6) | 0.827 | −3.4 (0.5) | < 0.001 | < 0.001 |
| % Calories from carbohydrates | |||||
| Baseline | 45.1 (0.7) | 46.8 (0.5) | 0.078 | ||
| Δ Month 12 | −0.7 (0.7) | 0.578 | 3.6 (0.5) | < 0.001 | < 0.001 |
| Δ Month 24 | −0.8 (0.7) | 0.482 | 2.4 (0.5) | < 0.001 | < 0.001 |
Baseline values are the observed mean (SE); change scores are the least‐squares adjusted means (SE) from the ITT repeated measures analysis.
Within‐group P‐value tests for a significant change from baseline to the follow‐up time point in that group; between‐group P‐value tests for a significant between‐group difference in the change score at the time point. All P‐values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text).
IGF‐1 axis and other growth factors
The substantial and sustained increase in serum IGFBP‐1 concentration and the reduction in IGF‐1:IGFBP‐1 ratio in CR significantly exceeded changes in AL (Table 4). Serum IGFBP‐3 reductions in CR significantly exceeded those in AL at 12 months (P = 0.018), but not at 24 months (Table 4). In contrast, serum IGF‐1, IGF‐1:IGFBP‐3 ratio and PDGF‐AB levels did not change significantly between groups at 12 and 24 months (Table 4). Serum TGF‐β1 decreased from baseline at 12 and 24 months in CR (Table 4), but the declines did not differ significantly from the change in AL. Serum cortisol concentration slightly but significantly increased in the CR group at 12 months (P = 0.003), but not at 24 months (Table 4).
Table 4.
Change from baseline in plasma concentrations of growth factors and cortisol at 12 and 24 months in AL and calorie restriction (CR) groups
| Outcome | AL | CR | Between‐group P‐valueb | ||
|---|---|---|---|---|---|
| Mean (SE)a | Within‐group P‐valueb | Mean (SE)a | Within‐group P‐valueb | ||
| IGF‐1 (ng mL−1) | |||||
| Baseline | 183.1 (5.7) | 175.5 (3.6) | 0.589 | ||
| Δ Month 12 | −19.6 (4.9) | < 0.001 | −7.1 (3.7) | 0.108 | 0.072 |
| Δ Month 24 | −18.7 (4.1) | < 0.001 | −15.1 (3.2) | < 0.001 | 0.919 |
| IGFBP‐1 (pg mL−1) | |||||
| Baseline | 4477 (585) | 5459 (523) | 0.088 | ||
| Δ Month 12 | 409 (636) | 1.0 | 1839 (474) | < 0.001 | 0.065 |
| Δ Month 24 | −616 (573) | 0.568 | 1391 (443) | 0.004 | 0.005 |
| IGFBP‐3 (ng mL−1) | |||||
| Baseline | 2528 (58.4) | 2459 (33) | 0.338 | ||
| Δ Month 12 | 1 (43) | 1.0 | 124 (32) | < 0.001 | 0.018 |
| Δ Month 24 | 56 (49) | 0.510 | 123 (38) | 0.003 | 0.273 |
| IGF‐1/IGFBP‐3 ratio | |||||
| Baseline | 0.10 (0.00) | 0.10 (0.00) | 0.942 | ||
| Δ Month 12 | −0.008 (0.002) | < 0.001 | −0.006 (0.002) | 0.001 | 0.880 |
| Δ Month 24 | −0.008 (0.002) | < 0.001 | −0.009 (0.002) | < 0.001 | 1.0 |
| IGF‐1/IGFBP‐1 ratio | |||||
| Baseline | 0.102 (0.012) | 0.078 (0.008) | 0.064 | ||
| Δ Month 12 | −0.007 (0.018) | 1.0 | −0.046 (0.014) | 0.002 | 0.088 |
| Δ Month 24 | −0.020 (0.008) | 0.018 | −0.045 (0.006) | < 0.001 | 0.008 |
| Cortisol (μg dL−1) | |||||
| Baseline | 11.3 (0.69) | 11.2 (0.41) | 0.667 | ||
| Δ Month 12 | −0.91 (0.46) | 0.102 | 0.78 (0.35) | 0.055 | 0.003 |
| Δ Month 24 | −1.78 (0.51) | 0.001 | −0.44 (0.39) | 0.530 | 0.312 |
| PDGF‐AB (pg mL−1) | |||||
| Baseline | 20 000 (699) | 18 131 (583) | 0.018 | ||
| Month 12 | −398 (628) | 1.0 | −26 (469) | 1.0 | 1.0 |
| Month 24 | −681 (515) | 0.375 | −1465 (398) | < 0.001 | 0.426 |
| TGF‐β1 (pg mL−1) | |||||
| Baseline | 30 604 (932) | 28 871 (813) | 0.065 | ||
| Δ Month 12 | −3169 (932) | 0.002 | −3521 (697) | < 0.001 | 1.0 |
| Δ Month 24 | −5455 (707) | < 0.001 | −6616 (549) | < 0.001 | 0.356 |
Baseline values are the observed mean (SE); change scores are the least‐squares adjusted means (SE) from the ITT repeated measures analysis.
Within‐group P‐value tests for a significant change from baseline to the follow‐up time point in that group; between‐group P‐value tests for a significant between‐group difference in the change score at the time point. All P‐values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text).
Discussion
Research on aging and cancer mechanisms has shown that the IGF pathway is deeply implicated in the biology of aging and in pathogenesis of several common malignant and potentially deadly tumors (e.g., colon, prostate, breast and ovarian cancer) (3) and that diet (e.g., CR) influences cancer risk through this pathway (Renehan et al., 2004; Fontana et al., 2010; Longo & Fontana, 2010; Guevara‐Aguirre et al., 2011). Most research data in this field have been derived from animal, epidemiological and observational studies, because it is difficult and expensive to conduct long‐term randomized clinical trials in humans. Thus, this is the first adequately powered randomized clinical trial to test in nonobese humans the long‐term effects of CR on the IGF‐1 axis and other growth factors implicated in the pathogenesis of cancer.
The findings of this clinical trial showed that, unlike in rodents (Breese et al., 1991), long‐term CR results in a significant and persistent increase in serum IGFBP‐1 and decrease in IGF‐1:IGFBP‐1 ratio levels, which should translate into lower circulating levels of free IGF‐1 and inhibition of IGF‐1 activity in humans. IGFBP‐1 concentrations were 25.2% and 21% higher after 1 and 2 years of CR, respectively, resulting in a 42% lower IGF‐1:IGFBP‐1 ratio level in the CR group at 2 years. This increase in serum IGFBP‐1 levels in our CR research volunteers may be mediated by improved insulin sensitivity (Suikkari et al., 1988). In fact, serum concentration of IGFBP‐1, unlike IGFBP‐3 which binds 75–90% of circulating IGF‐I, is heavily influenced by the metabolic (i.e., insulin resistance, and insulin and glucagon levels) and nutritional (fasting and refeeding) state of the individual. Excessive adiposity‐induced insulin resistance and compensatory hyperinsulinemia have been shown to decrease hepatic synthesis of IGFBP‐1, which translates into increased concentrations of bioavailable IGF‐1, without modifications in serum total IGF‐1 levels (Lukanova et al., 2001; Maddux et al., 2006). Patients with type 1 diabetes have higher serum IGFBP‐1 concentrations than normoglycemic controls (Suikkari et al., 1988), and acute steady state hyperinsulinemia lowers serum IGFBP‐1 levels by 40–70% in normal individuals (Yeoh & Baxter, 1988; Snyder & Clemmons, 1990). Moreover, it has been shown that circulating levels of IGFBP‐1 are acutely increased by 3–4 fold in response to overnight fasting and decline rapidly after a meal (Busby et al., 1988; Smith et al., 1995).
Fasting, but not long‐term CR, lowers serum IGF‐1 concentration in humans into the range observed for growth hormone‐deficient patients (Thissen et al., 1994; Fontana et al., 2008; Redman et al., 2010). Consistent with these previous human studies, we found that, unlike in rodents, the reduction in energy intake in the CR group was not accompanied by a decrease in serum IGF‐1 concentration or IGF‐1/IGFBP‐3 ratio, even in younger men and women. One possible explanation for the lack of effect of CR on IGF‐1 levels in this and other studies may be the chronic high‐normal intake of dietary proteins, which was two‐fold higher than the RDA median (i.e., 0.6 g kg−1 day−1). In a previous study, we have shown that reducing protein intake from 1.6 to 0.95 g kg−1 day−1 was necessary to reduce serum IGF‐1 concentrations in individuals practicing severe CR (Fontana et al., 2008). We also found that protein restriction, independently of caloric intake, strongly inhibits tumor growth in human xenograft prostate and breast cancer animal models, by lowering serum IGF‐1 and reducing mTOR phosphorylation (Fontana et al., 2013). Consistently, it has been shown that in humans the variations of circulating IGF‐1 levels during fasting and refeeding are strongly linked with the rate of urinary urea excretion, a well‐known indicator of protein intake (Clemmons et al., 1981).
In this study, we also measured two growth factors, TGF‐β‐1 and PDGF‐AB, implicated in the pathogenesis of cancer (Silver, 1992; Meulmeester & Ten Dijke, 2011), which have previously been shown in observational studies to be reduced by long‐term severe CR in humans (Fontana et al., 2004; Meyer et al., 2006). Our findings confirmed that CR induces a significant reduction in serum TGF‐β‐1 and PDGF‐AB over time, but the differences between groups were not significant. Data from animal studies have shown that increased serum corticosterone concentration may also play a role in the CR‐induced protective effects against cancer, as adrenalectomy completely reverses the tumor inhibitory effect of CR in some studies (Pashko & Schwartz, 1992, 1996; Stewart et al., 2005). It has been hypothesized that a CR‐induced moderate increase in glucocorticoid concentrations may influence cancer development by inhibiting inflammation and promoting the activation of molecular chaperones (e.g., heat‐shock proteins), which ensure proteostasis (Sapolsky et al., 2000; Rhen & Cidlowski, 2005). Data from two previous small, short‐term studies have shown that CR‐induced weight loss does not increase cortisol concentration in overweight men and women (Weiss et al., 2006; Tam et al., 2014). In contrast, this study shows that 1 year of CR slightly but significantly increases serum cortisol level in young and middle‐aged lean or slightly overweight men and women. This ~6% elevation in cortisol, however, was transient because no change in serum cortisol concentration was detected after 2 years of CR.
In conclusion, data from this large randomized clinical trial showed that long‐term CR does not reduce serum IGF‐1 concentration, but causes a reduction in IGF‐1 bioavailability by substantially and persistently increasing IGFBP‐1 levels in young and middle‐aged lean or slightly overweight men and women. These data also showed that long‐term CR results in mild and transient increase in serum cortisol concentrations. More studies are needed to understand the biological implications of these metabolic adaptations on cancer risk, health, and longevity, and whether or not other nutritional interventions (e.g., protein restriction or intermittent fasting) reduce serum IGF1 concentration in humans.
Experimental procedures
Figure 1 shows the CONSORT diagram which has been reported previously (Ravussin et al., 2015). Briefly, CALERIE was an intensive multicenter randomized, controlled trial with the aim of determining the effects of 25% CR (a reduction in energy intake to 25% below the individual's baseline level) over a 2‐year period in healthy volunteers. Men were required to be between 20 and 50 years, and women between 20 and 47 years, of age, with a BMI between 22 and 27.9 kg m−2. The study protocol (NCT00427193) was approved by the Institutional Review Boards of Washington University (St. Louis, MO, USA), Tufts University (Boston, MA, USA), Pennington Biomedical Research Center (Baton Rouge, LA, USA), and Duke University (Durham, NC, USA). Study oversight was provided by a Data and Safety Monitoring Board. The participants provided written informed consent.
Treatment assignment and intervention
After baseline testing, volunteers were randomized to 25% CR or ad libitum control groups with a 2:1 allocation in favor of the CR group. Randomization was stratified for site, sex, and BMI. The CR participants were prescribed a 25% reduction in energy intake based on energy requirements determined by doubly labeled water measurements over a 4‐week period. Adherence to the prescribed 25% CR was calculated by the degree to which the study volunteers attained a prescribed weight loss trajectory (average at 1‐year 15.5%, range of 11.9–22.1%) followed by weight maintenance. Furthermore, the accurate level of CR was retrospectively validated by measuring the total daily energy expenditure (TDEE) by doubly labeled water and adjusting TDEE for changes in body composition (Racette et al., 2012).
Body weight and body composition
Body weight was measured fasting in the morning in a light gown. Fat mass (FM) and fat‐free mass (FFM) were measured by DXA using Hologic 4500A, Delphi W or Discovery A scanners (Hologic QDR 4500A; Hologic, Bedford, MA). Scans were analyzed at University of California San Francisco, also responsible for centralized quality control. Machine performance was monitored with baseline and longitudinal phantom cross‐calibrations.
Dietary intake
Dietary intakes were determined using 6‐day food diaries which were analyzed with Nutrition Data System for Research (Minneapolis, MN, USA).
Assays
Serum blood samples were obtained after fasting overnight and analyzed in a central laboratory. Commercially available ELISA kits were used to measure IGF‐1, IGF‐binding protein 1, (IGFBP‐1) and IGFBP‐3 (DSL/Beckman Coulter, Brea, CA, USA), and PDGF‐AB and TGF‐β‐1 (R&D Systems, Minneapolis, MN, USA). Cortisol was measured by chemiluminescent immunoassay (ADVIA Centaur, Bayer Health Care, Deerfield, IL).
Statistical analysis
The same statistical methodologies used in the parent RCT were applied (Racette et al., 2012). Briefly, Wilcoxon and the Fisher exact tests were used to evaluate between‐group differences with respect to baseline characteristics. Repeated measures analysis of covariance was applied with change from baseline as the dependent variable, and treatment, time, and the treatment × time interaction as independent variables; site, sex, BMI stratum, and the baseline value were included as covariates. Hypotheses of specific interest, for example, between‐group differences at the individual time points and within‐group changes over time were tested by defining contrasts among the regression parameters; predicted mean change ± standard error are the adjusted values from this model. For any outcome, type‐I error was controlled using a hierarchical gatekeeping strategy (Dmitrienko et al., 2011). The treatment × visit interaction term was tested first. If significant, then following standard statistical practice, between‐group differences at each time point were tested at α = 0.05. If not, the treatment main effect was tested next. If significant, then between‐group differences at each time point were tested at α = 0.05. Otherwise a Bonferroni correction was applied at each time point, with the P‐values adjusted by multiplying the nominal P‐value by the number of tests (truncated at 1.0) (Wright, 1992). Within‐group changes from baseline to the follow‐up visits, however, fell outside this hierarchy and were always protected by a Bonferroni correction.
Funding source
This study was supported by National Institute on Aging Cooperative Agreements U01‐AG‐020487, U01‐AG‐020478, U01‐AG‐020480, and U01‐AG‐022132 and National Institutes of Health Grants MO1‐RR00036, P30‐DK‐056341 and UL1RR024992.
Author contributions
The Corresponding Author attests that the authors had access to all the study data, takes responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Conflict of interest
The authors hereby declare that there was no conflict of interest associated with this study.
Acknowledgments
Consistent with an NIH cooperative agreement, the study was designed and managed by a Steering Committee consisting of the Principal Investigators of the three clinical sites and the Data Coordinating Center, and a representative from the NIA. Decisions were made by majority vote and all members, including the NIH, had one vote.
Registration: ClinicalTrials.gov number: NCT00427193
References
- Albanes D (1987) Total calories, body weight, and tumor incidence in mice. Cancer Res. 47, 1987–1992. [PubMed] [Google Scholar]
- Bartke A, Sun LY, Longo V (2013) Somatotropic signaling: trade‐offs between growth, reproductive development, and longevity. Physiol. Rev. 93, 571–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Ingram RL, Sonntag WE (1991) Influence of age and long‐term dietary restriction on plasma insulin‐like growth factor‐1 (IGF‐1), IGF‐1 gene expression, and IGF‐1 binding proteins. J. Gerontol. 46, B180–B187. [DOI] [PubMed] [Google Scholar]
- Busby WH, Snyder DK, Clemmons DR (1988) Radioimmunoassay of a 26,000‐dalton plasma insulin‐like growth factor‐binding protein: control by nutritional variables. J. Clin. Endocrinol. Metab. 67, 1225–1230. [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Klibanski A, Underwood LE, McArthur JW, Ridgway EC, Beitins IZ, Van Wyk JJ (1981) Reduction of plasma immunoreactive somatomedin C during fasting in humans. J. Clin. Endocrinol. Metab. 53, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrienko A, Millen BA, Brechenmacher T, Paux G (2011) Development of gatekeeping strategies in confirmatory clinical trials. Biom. J. 53, 875–893. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC (1997) Dietary restriction reduces insulin‐like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53‐deficient mice. Cancer Res. 57, 4667–4672. [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO (2004) Long‐term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA 10, 6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO (2008) Long‐term effects of calorie or protein restriction on serum IGF‐1 and IGFBP‐3 concentration in humans. Aging Cell 7, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD (2010) Extending healthy life span–from yeast to humans. Science 328, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Adelaiye RM, Rastelli AL, Miles KM, Ciamporcero E, Longo VD, Nguyen H, Vessella R, Pili R (2013) Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget 4, 2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara‐Aguirre J, Balasubramanian P, Guevara‐Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin‐Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD (2011) Growth hormone receptor deficiency is associated with a major reduction in pro‐aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 3, 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick JJ, List EO, Kelder B, Gosney ES, Berryman DE (2014) Evaluation of growth hormone (GH) action in mice: discovery of GH receptor antagonists and clinical indications. Mol. Cell. Endocrinol. 386, 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Fontana L (2010) Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol. Sci. 31, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukanova A, Toniolo P, Akhmedkhanov A, Hunt K, Rinaldi S, Zeleniuch‐Jacquotte A, Haley NJ, Riboli E, Stattin P, Lundin E, Kaaks R (2001) A cross‐sectional study of IGF‐I determinants in women. Eur. J. Cancer Prev. 10, 443–452. [DOI] [PubMed] [Google Scholar]
- Maddux BA, Chan A, De Filippis EA, Mandarino LJ, Goldfine ID (2006) IGF‐binding protein‐1 levels are related to insulin‐mediated glucose disposal and are a potential serum marker of insulin resistance. Diabetes Care 29, 1535–1537. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R (2012) Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulmeester E, Ten Dijke P (2011) The dynamic roles of TGF‐b in cancer. J. Pathol. 223, 205–218. [DOI] [PubMed] [Google Scholar]
- Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L (2006) Long‐term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Coll. Cardiol. 47, 398–402. [DOI] [PubMed] [Google Scholar]
- Pashko LL, Schwartz AG (1992) Reversal of food restriction induced inhibition of mouse skin tumor promotion by adrenalectomy. Carcinogenesis 13, 1925–1928. [DOI] [PubMed] [Google Scholar]
- Pashko LL, Schwartz AG (1996) Inhibition of 7,12‐dimethylbenz‐[a]anthracene‐induced lung tumorigenesis in A/J mice by food restriction is reversed by adrenalectomy. Carcinogenesis 17, 209–212. [DOI] [PubMed] [Google Scholar]
- Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, Pieper C, DeLany JP, Kraus WE, Rochon J, Redman LM, CALERIE Study Group (2012) Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am. J. Physiol. Endocrinol. Metab. 302, E441–E448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB, CALERIE Study Group (2015) A 2‐Year Randomized Controlled Trial of Human Caloric Restriction: feasibility and Effects on Predictors of Health Span and Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015; 70, 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Veldhuis JD, Rood J, Smith SR, Williamson D, Ravussin E, Pennington CALERIE Team (2010) The effect of caloric restriction interventions on growth hormone secretion in nonobese men and women. Aging Cell 9, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan AG, O'Connell J, O'Halloran D, Shanahan F, Potten CS, O'Dwyer ST, Shalet SM (2003) Acromegaly and colorectal cancer: a comprehensive review of epidemiology, biological mechanisms, and clinical implications. Horm. Metab. Res. 35, 712–725. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M (2004) Insulin‐like growth factor (IGF)‐I, IGF binding protein‐3, and cancer risk: systematic review and meta‐regression analysis. Lancet 363, 1346–1353. [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA (2005) Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723. [DOI] [PubMed] [Google Scholar]
- Sabatino F, Masoro EJ, McMahan CA, Kuhn RW (1991) Assessment of the role of the glucocorticoid system in aging processes and in the action of food restriction. J. Gerontol. 46, B171–B179. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. [DOI] [PubMed] [Google Scholar]
- Shevah O, Laron Z (2007) Patients with congenital deficiency of IGF‐I seem protected from the development of malignancies: a preliminary report. Growth Horm. IGF Res. 17, 54–57. [DOI] [PubMed] [Google Scholar]
- Silver BJ (1992) Platelet‐derived growth factor in human malignancy. BioFactors 3, 217–227. [PubMed] [Google Scholar]
- Smith WJ, Underwood LE, Clemmons DR (1995) Effects of calorie or protein restriction on insulin‐like growth factor‐I (IGF‐1) and IGF‐binding proteins in children and adults. J. Clin. Endocrinol. Metab. 80, 443–449. [DOI] [PubMed] [Google Scholar]
- Snyder DK, Clemmons DR (1990) Insulin‐dependent regulation of insulin‐like growth factor‐binding protein‐1. J. Clin. Endocrinol. Metab. 71, 1632–1636. [DOI] [PubMed] [Google Scholar]
- Stewart JW, Koehler K, Jackson W, Hawley J, Wang W, Au A, Myers R, Birt DF (2005) Prevention of mouse skin tumor promotion by dietary energy restriction requires an intact adrenal gland and glucocorticoid supplementation restores inhibition. Carcinogenesis 26, 1077–1084. [DOI] [PubMed] [Google Scholar]
- Suikkari AM, Koivisto VA, Rutanen EM, Yki‐Jarvinen H, Karonen SL, Seppala M (1988) Insulin regulates the serum levels of low molecular weight insulin‐like growth factor‐binding protein. J. Clin. Endocrinol. Metab. 66, 266–272. [DOI] [PubMed] [Google Scholar]
- Tam CS, Frost EA, Xie W, Rood J, Ravussin E, Redman LM, Pennington CALERIE Team (2014) No effect of caloric restriction on salivary cortisol levels in overweight men and women. Metabolism 63, 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thissen JP, Ketelslegers JM, Underwood LE (1994) Nutritional regulation of the insulin‐like growth factors. Endocr. Rev. 1, 80–101. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger‐May K, Schechtman KB, Klein S, Holloszy JO, Washington University School of Medicine CALERIE Group (2006) Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am. J. Clin. Nutr. 84, 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SP (1992) Adjusted p‐values for simultaneous inference. Biometrics 48, 1005–1013. [Google Scholar]
- Yakar S, Leroith D, Brodt P (2005) The role of the growth hormone/insulin‐like growth factor axis in tumor growth and progression: lessons from animal models. Cytokine Growth Factor Rev. 16, 407–420. [DOI] [PubMed] [Google Scholar]
- Yeoh SI, Baxter RC (1988) Metabolic regulation of the growth hormone independent insulin‐like growth factor binding protein in human plasma. Acta Endocrinol. (Copenh) 119, 465–473. [DOI] [PubMed] [Google Scholar]
- Yu H, Rohan T (2000) Role of the insulin‐like growth factor family in cancer development and progression. J. Natl Cancer Inst. 92, 1472–1489. [DOI] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B (2009) Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]


