Figure 6.
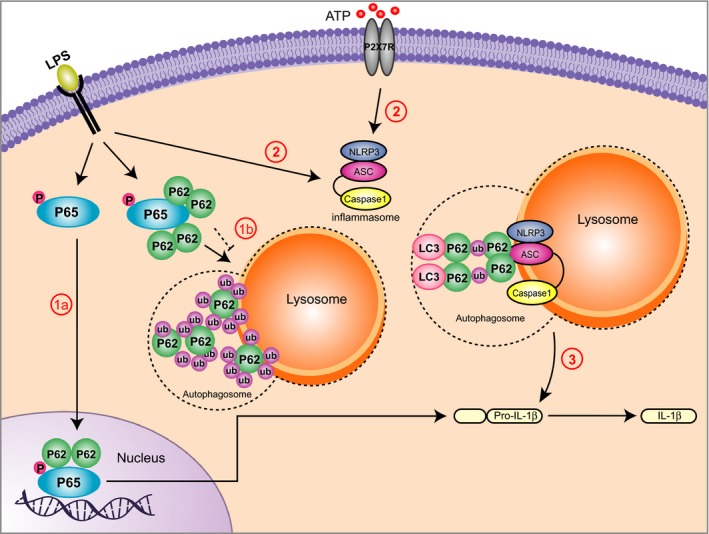
Inflammasome activation due to impaired autophagy in Gaucher macrophages (GMs) (1) In both control and GMs, lipopolysaccharide (LPS) priming induces activation of p65‐NF‐kB, which is translocated to the nucleus, leading to production of pro‐IL‐1β in the cytosol. In control macrophages (1a), LPS stimulates accumulation of ubiquitinated p65‐NF‐kB, which is further recognized by p62, delivered to autophagosomes, and degraded in the lysosome (1a, solid line). In GMs (1b, dashed line), impaired autophagy prevents degradation of p65‐ NF‐kB through autophagy and results in its activation (1b). (2) In both control and GMs, stimulation by both LPS and extracellular ATP leads to inflammasome complex formation (NLRP3, apoptosis‐associated speck‐like proteins (ASC) and caspase1) and activation. (3) Activated inflammasomes undergo ubiquitination of ASC, leading to p62‐mediated engulfment of inflammasomes by autophagosomes. Pro‐IL‐1β conversion to active IL‐1β is limited due to the destruction of activated inflammasomes by autophagolysosomes. In GMs, defective autophagy and lysosomal dysfunction inhibit the elimination of active inflammasomes through autophagy, resulting in the upregulation and secretion of IL‐1β (3).
