Abstract
Non-tuberculous mycobacteria (NTM) are ubiquitous environmental organisms that can cause chronic pulmonary infection, particularly in individuals with pre-existing inflammatory lung disease such as cystic fibrosis (CF). Pulmonary disease caused by NTM has emerged as a major threat to the health of individuals with CF but remains difficult to diagnose and problematic to treat. In response to this challenge, the US Cystic Fibrosis Foundation (CFF) and the European Cystic Fibrosis Society (ECFS) convened an expert panel of specialists to develop consensus recommendations for the screening, investigation, diagnosis and management of NTM pulmonary disease in individuals with CF. Nineteen experts were invited to participate in the recommendation development process. Population, Intervention, Comparison, Outcome (PICO) methodology and systematic literature reviews were employed to inform draft recommendations. An anonymous voting process was used by the committee to reach consensus. All committee members were asked to rate each statement on a scale of: 0, completely disagree, to 9, completely agree; with 80% or more of scores between 7 and 9 being considered ‘good’ agreement. Additionally, the committee solicited feedback from the CF communities in the USA and Europe and considered the feedback in the development of the final recommendation statements. Three rounds of voting were conducted to achieve 80% consensus for each recommendation statement. Through this process, we have generated a series of pragmatic, evidence-based recommendations for the screening, investigation, diagnosis and treatment of NTM infection in individuals with CF as an initial step in optimising management for this challenging condition.
Keywords: Cystic Fibrosis, Bacterial Infection
Background
Epidemiology of non-tuberculous mycobacteria in individuals with cystic fibrosis
Non-tuberculous mycobacteria (NTM) are increasingly being isolated from the sputum of adults and children with cystic fibrosis (CF), both in North America and in Europe.1–17 Estimates of the prevalence of NTM in the CF population have ranged from 1.3% in the earliest study reported in 19841 to 32.7% in a review of individuals with CF over the age of 40 years in Colorado.9 To date, the largest studies published examined 986,6 121615 and 158217 individuals with CF and reported rates of NTM-positive cultures of 13.0%, 13.7% and 6.6%, respectively. Recently, analysis of US Cystic Fibrosis Foundation (CFF) registry data has shown prevalence rates for NTM-positive culture in the USA of 12%18 but with considerable variation between individual states (0–28%).19
The NTM species most commonly identified in individuals with CF from North America and Europe are the slow-growing Mycobacterium avium complex (MAC) (including M. avium, M. intracellulare and M. chimaera), which can be found in up to 72% of NTM-positive sputum cultures,6 and the rapid-growing M. abscessus complex (MABSC) (comprising the subspecies M. abscessus subsp abscessus (M. a. abscessus), M. a. bolletii20 and M. a. massiliense21 22 (the latter currently classified as part of M. a. bolletii)), which in many centres has now become the most common NTM isolated from individuals with CF.7 15 17 21 23–25 Other less commonly isolated species include M. simiae,11 M. kansasii and M. fortuitum.26 There are geographical differences in both the prevalence of NTM-positive cultures and also the relative frequency of different species seen between but also within countries.6 17 19 24 25 27
NTM acquisition is strongly associated with age in individuals with CF, with prevalence increasing from 10% in children aged 10 years, to over 30% in adults over the age of 40 years.9 In individuals with an adult diagnosis of CF, over 50% (mostly females) have NTM-positive airway cultures.9 There appear to be species-specific differences in age-related prevalence within CF cohorts, with MAC more commonly isolated from adults over 25 years of age,6 7 14 17 27 while MABSC is isolated from all age groups, but peaks between those 11 and 15 years of age in some studies.17 28 There may also be species-specific differences in virulence: individuals with MABSC-positive cultures are more likely to meet American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) criteria for diagnosing NTM pulmonary disease (NTM-PD, see Diagnosis of NTM-PD in CF section), and have worse morbidity and mortality associated with a more rapid decline in lung function.15 27 29 30
There has been a rise over the last four decades in the reported prevalence of NTM-positive cultures in respiratory samples from individuals with CF,1 6 15 17 18 23 an increase in part mirroring temporal changes seen in the non-CF cohort.31–38 While increasing detection rates may reflect enhanced surveillance and/or improved microbiological detection,6 27 39–42 there are a number of lines of evidence suggesting a true rise in the frequency of NTM infection. A number of CF studies43 show year on year increases in NTM-positive cultures with no change in surveillance intensity or culture methodology. There has been an increase over time in rates of skin test reactivity to NTM antigens in US population-based testing studies,44 potentially indicating increasing exposure to NTM (see below). Furthermore, the relative frequency of M. abscessus detection in NTM-positive samples from individuals with CF has increased remarkably over time both in the USA and in Europe,2 6 15 17 23 27 suggesting real changes in NTM acquisition rates (rather than increased sampling).
Possible reasons for the potential increased frequency of NTM-positive cultures in individuals with CF include: increases in environmental exposure to NTM through more permissive temperature settings of home water heaters45 and more contact with shower aerosols,46 47 increased antibiotic usage creating more NTM favourable lung niches,27 greater chronic use of medications that might impair host immunity to NTM,43 and/or spread of NTM through person-to-person transmission.48 49
NTM-PD in individuals with CF
NTM can cause progressive inflammatory lung damage, a condition termed ‘NTM pulmonary disease’ (NTM-PD),50 51 which is defined by the presence of specific microbiological, clinical and radiological features described in Diagnosis of NTM-PD in CF section. However, it has become clear that NTM can also transiently, intermittently or permanently reside within the lungs of individuals with CF without causing NTM-PD, thus representing asymptomatic infection and creating considerable difficulties in deciding how best to screen for and diagnose NTM.30 Further challenges exist in knowing how best to identify NTM in respiratory samples, when and how to initiate treatment for NTM-PD (as highlighted by a recent Cochrane review52) and how NTM may impact individuals under consideration for lung transplantation. As a consequence, the CFF and European Cystic Fibrosis Society (ECFS) sought to generate a consensus recommendations document to support and standardise the management of NTM infection in individuals with CF, permitting prospective evaluation of current best practice and forming a foundation for future research programmes.
These consensus statements have been developed to assist in the management both of adults and children with CF who are infected with NTM. Given the virtual absence of published evidence to guide paediatric care,53 recommendations for children with CF infected with NTM are based on extrapolated adult data, the practical experience of experts and appropriate adjustment of drug regimens, and are, except where stated, the same as for adults.
Methods
Expert committee structure
The CFF and the ECFS invited experts to participate in the statement development process. The 19-member committee consisted of professionals (10 US and 9 European) with expertise in CF and NTM, and included adult and paediatric CF physicians, lung transplant physicians, microbiologists, infectious disease specialists and a parent of an individual with CF. The committee convened in May 2012 and was divided into five subgroups, each responsible for a specific topic: Epidemiology and Risk Factors, Screening, Microbiology, Treatment and Transplantation. Each subgroup developed topic-specific questions using the PICO format (Population, Intervention, Comparison, Outcome.54) Questions were reviewed and approved by the entire committee before systematic literature searches were conducted.
Review process and consensus vote
The members of each subgroup used the PICO questions to guide literature searches in PubMed. Searches were limited to the English language and the period 1984 to 2013. Subgroup members also searched for topic-relevant guidelines through searches of the ATS website, the IDSA website, the Clinical Laboratory Standards Institute (CLSI) website and the UK CF Trust website.
After reviewing the relevant literature and existing guidelines, subgroup members drafted recommendation statements. In October 2012, a second meeting was convened and subgroups finalised draft recommendation statements. The committee also voted to set 80% agreement of all 19 members as the threshold for acceptance of a recommendation statement and not to use the GRADE system of evaluating published evidence, given the paucity of clinical trial data.
Each subgroup submitted final draft questions for entry into an electronic survey tool (Survey Monkey) for the purposes of anonymous voting and comment by all members. A project coordinator administered the survey and committee members were asked to rate each statement on a scale of: 0, completely disagree, to 9, completely agree; with 80% or between 7 and 9 being considered ‘good’ agreement. Space for entering free text was also provided after each statement to allow members to cite literature in support of their opinions or suggested revisions. All committee members were required to vote on each statement regardless of their role or expertise. Multiple rounds of voting and revisions to the statements were conducted, and for each round committee members were requested to complete their voting within 3 weeks. The committee chairs reviewed the results from each round and updated the statements based on comments entered by respondents for subsequent rounds.
External review
A draft of the recommendations was presented at the 2013 North American Cystic Fibrosis Conference and the European Cystic Fibrosis Society Meeting. Additionally, the committee solicited feedback from the CF communities in the USA and in Europe, which included physicians, nurses, physical and respiratory therapists, parents and individuals with CF. All comments collected from this process were reviewed and addressed by the committee in the development of the final recommendation statements.
Results
Final recommendations and results of the consensus vote
Three rounds of voting were conducted to achieve 80% consensus for each statement. Fifty-three statements were included in the first round of voting and 50 statements in the second and third rounds. Final statements and the consensus are reported in table 1.
Table 1.
NTM recommendation statements
| Recommendation | Consensus (%) |
|---|---|
| Recommendation 1: The CF Foundation and the ECFS recommend that the potential for cross-infection of NTM (particularly Mycobacterium abscessus complex) between individuals with CF should be minimised by following national infection control guidelines | 94 |
| Recommendation 2: The CF Foundation and the ECFS recommend that cultures for NTM be performed annually in spontaneously expectorating individuals with a stable clinical course | 94 |
| Recommendation 3: The CF Foundation and the ECFS recommend that, in the absence of clinical features suggestive of NTM pulmonary disease, individuals who are not capable of spontaneously producing sputum do not require screening cultures for NTM | 100 |
| Recommendation 4: The CF Foundation and the ECFS recommend that culture and smears for AFB from sputum should be used for NTM screening | 100 |
| Recommendation 5: The CF Foundation and the ECFS recommend against the use of oropharyngeal swabs for NTM screening | 100 |
| Recommendation 6: The CF Foundation and the ECFS recommend that culture and smears for AFB from sputum, induced sputum, bronchial washings or bronchoalveolar lavage samples can be used to evaluate individuals with CF suspected to have NTM pulmonary disease. | 100 |
| Recommendation 7: The CF Foundation and the ECFS recommend against the routine use of transbronchial biopsies to detect NTM in individuals with CF suspected to have NTM pulmonary disease | 100 |
| Recommendation 8: The CF Foundation and the ECFS recommend against the use of oropharyngeal swabs to perform diagnostic smears and cultures in individuals with CF suspected to have NTM pulmonary disease | 100 |
| Recommendation 9: The CF Foundation and the ECFS recommend that respiratory tract samples should be cultured using both solid and liquid media | 100 |
| Recommendation 10: The CF Foundation and the ECFS recommend that the incubation duration for NTM cultures should be for a minimum of 6 weeks | 100 |
| Recommendation 11: The CF Foundation and the ECFS recommend that an NTM culture should be processed within 24 h of collection to optimise the detection of NTM in respiratory samples. If a delay in processing is anticipated, refrigeration of samples is advised | 100 |
| Recommendation 12: The CF Foundation and the ECFS recommend that respiratory tract samples should be decontaminated using the standard N-acetyl l-cysteine, NALC, (0.5%)–NaOH (2%) method | 100 |
| Recommendation 13: The CF Foundation and the ECFS recommend that, if a sample remains contaminated with Gram-negative bacteria after standard NALC-NaOH decontamination, it should be further treated with either 5% oxalic acid or 1% chlorhexidine | 100 |
| Recommendation 14: The CF Foundation and the ECFS recommend against the use of non-culture-based methods for detecting NTM in respiratory tract samples | 100 |
| Recommendation 15: The CF Foundation and the ECFS recommend that all NTM isolates from individuals with CF should undergo molecular identification | 100 |
| Recommendation 16: The CF Foundation and the ECFS recommend that all NTM isolates from individuals with CF should be identified to the species level, except for M. intracellulare, M. avium and M. chimaera, where identification can be limited to MAC, and M. abscessus complex, which should be subspeciated | 83 |
| Recommendation 17: The CF Foundation and the ECFS recommend that for MAC, clarithromycin susceptibility testing should be performed on an isolate recovered prior to initiation of treatment. Clarithromycin susceptibility testing should also be performed on subsequent isolates if the patient (a) fails to culture convert after 6 months of NTM treatment; (b) recultures MAC after initial culture conversion while on NTM treatment or (c) recultures MAC after completion of NTM treatment | 94 |
| Recommendation 18: The CF Foundation and the ECFS recommend that for M. abscessus complex, susceptibility testing should include at least clarithromycin, cefoxitin and amikacin (and preferably also tigecycline, imipenem, minocycline, moxifloxacin and linezolid) | 89 |
| Recommendation 19: The CF Foundation and the ECFS recommend that drug susceptibility testing should be performed in accordance with CLSI guidelines | 100 |
| Recommendation 20: The CF Foundation and the ECFS recommend that ATS/IDSA criteria for the diagnosis of NTM pulmonary disease should be used in individuals with CF (ATS/IDSA 2007 Statement) | 100 |
| Recommendation 21: The CF Foundation and the ECFS recommend that other CF pathogens and comorbidities should be considered as potential contributors to a patient's symptoms and radiological features when determining the clinical significance of NTM-positive cultures | 100 |
| Recommendation 22: The CF Foundation and the ECFS recommend that NTM treatment should be considered for individuals with CF who have ATS/IDSA defined NTM pulmonary disease | 100 |
| Recommendation 23: The CF Foundation and the ECFS recommend that individuals receiving azithromycin as part of their CF medical regimen who have a positive NTM culture should not continue azithromycin treatment while evaluation for NTM disease is underway as azithromycin monotherapy may lead to resistance. A macrolide agent may be included in a multidrug treatment regimen if criteria are met for NTM disease | 89 |
| Recommendation 24: The CF Foundation and the ECFS recommend that treatment of M. abscessus complex pulmonary disease should involve an intensive phase followed by a continuation phase | 100 |
| Recommendation 25: The CF Foundation and the ECFS recommend that the intensive phase should include a daily oral macrolide (preferably azithromycin) in conjunction with 3–12 weeks of intravenous amikacin and one or more of the following: intravenous tigecycline, imipenem or cefoxitin, guided but not dictated by drug susceptibility testing. The duration of intensive phase therapy should be determined by the severity of infection, the response to treatment and the tolerability of the regimen | 83 |
| Recommendation 26: The CF Foundation and the ECFS recommend that the continuation phase should include a daily oral macrolide (preferably azithromycin) and inhaled amikacin, in conjunction with 2–3 of the following additional oral antibiotics: minocycline, clofazimine, moxifloxacin and linezolid, guided but not dictated by drug susceptibility testing | 89 |
| Recommendation 27: The CF Foundation and the ECFS recommend that individuals with M. abscessus complex pulmonary disease should be managed in collaboration with experts in the treatment of NTM and CF, as drug intolerance and drug-related toxicity occur frequently, and changes in antibiotic therapy are often required | 89 |
| Recommendation 28: The CF Foundation and the ECFS recommend that monotherapy with a macrolide or other antimicrobial should never be used in the treatment of M. abscessus complex pulmonary disease | 100 |
| Recommendation 29: The CF Foundation and the ECFS recommend the same antibiotic regimen for treatment of all species within the MAC | 94 |
| Recommendation 30: The CF Foundation and the ECFS recommend that clarithromycin-sensitive MAC pulmonary disease should be treated with a daily oral antibiotic regimen containing a macrolide (preferably azithromycin), rifampin and ethambutol | 89 |
| Recommendation 31: The CF Foundation and the ECFS recommend against the use of intermittent (three times per week) oral antibiotic therapy to treat MAC pulmonary disease | 89 |
| Recommendation 32: The CF Foundation and the ECFS recommend that monotherapy with a macrolide or other antimicrobial agent should never be used in the treatment of MAC pulmonary disease | 100 |
Recommendation 33: The CF Foundation and the ECFS recommend that an initial course of intravenous amikacin should be considered for the treatment of MAC pulmonary disease in the presence of one or more of the following:
|
94 |
| Recommendation 34: The CF Foundation and the ECFS recommend that clarithromycin-resistant MAC pulmonary disease should be managed in collaboration with experts in the treatment of NTM and CF | 89 |
| Recommendation 35: The CF Foundation and the ECFS recommend that individuals with CF receiving NTM treatment should have expectorated or induced sputum samples sent for NTM culture every 4–8 weeks throughout the entire course of treatment to assess the microbiological response | 94 |
| Recommendation 36: The CF Foundation and the ECFS recommend that a schedule for detecting drug toxicity (including hearing loss, visual loss, renal impairment and liver function test abnormalities) should be set in place at the time of NTM treatment initiation and implemented throughout treatment based on the specific drugs prescribed | 100 |
| Recommendation 37: The CF Foundation and the ECFS recommend that an HRCT scan of the lungs should be performed shortly before starting NTM treatment and at the end of NTM treatment to assess the radiological response | 94 |
| Recommendation 38: The CF Foundation and the ECFS recommend that NTM antibiotic therapy should be prescribed for 12 months beyond culture conversion (defined as three consecutive negative cultures, with the time of conversion being the date of the first of the three negative cultures) as long as no positive cultures are obtained during those 12 months | 94 |
| Recommendation 39: The CF Foundation and the ECFS recommend that individuals who fail to culture convert despite optimal NTM therapy may benefit from long-term suppressive antibiotic treatment | 94 |
| Recommendation 40: The CF Foundation and the ECFS recommend that, when amikacin is given intravenously or when streptomycin is given intravenously or intramuscularly, serum levels should be monitored and dosing adjusted to minimise ototoxicity and nephrotoxicity | 100 |
| Recommendation 41: The CF Foundation and the ECFS recommend against routinely obtaining serum levels of other anti-mycobacterial drugs. However, absorption of oral medications is often reduced in CF. Therefore use of therapeutic drug monitoring should be considered for individuals failing to improve despite taking recommended drug regimens or for those on concomitant medications with significant interactions with NTM drugs | 100 |
| Recommendation 42: The CF Foundation and the ECFS recommend against the use of interferon γ as adjuvant therapy for NTM pulmonary disease in individuals with CF | 89 |
| Recommendation 43: The CF Foundation and the ECFS recommend that vitamin D should be supplemented according to national CF care guidelines | 94 |
| Recommendation 44: The CF Foundation and the ECFS recommend that lung resection should only be considered under extraordinary circumstances and in consultation with experts on the treatment of NTM and CF | 83 |
| Recommendation 45: The CF Foundation and the ECFS recommend that all individuals with CF being considered for lung transplantation should be evaluated for NTM pulmonary disease | 100 |
| Recommendation 46: The CF Foundation and the ECFS recommend that the presence of current or previous respiratory tract samples positive for NTM should not preclude individuals being considered for lung transplantation | 94 |
| Recommendation 47: The CF Foundation and the ECFS recommend that individuals with CF who have NTM pulmonary disease and are being evaluated for transplantation should start treatment prior to transplant listing | 100 |
| Recommendation 48: The CF Foundation and the ECFS recommend that individuals with CF receiving NTM treatment with sequential negative cultures may be eligible for transplant listing | 100 |
| Recommendation 49: The CF Foundation and the ECFS recommend that individuals with CF who have completed treatment for NTM pulmonary disease with apparent eradication of the organism may be eligible for transplant listing | 100 |
| Recommendation 50: The CF Foundation and the ECFS recommend that the presence of persistent M. abscessus complex or MAC infection despite optimal therapy is not an absolute contraindication to lung transplant referral | 94 |
AFB, acid-fast bacilli; CF, cystic fibrosis; CLSI, Clinical Laboratory Standards Institute; ECFS, European Cystic Fibrosis Society; HRCT, High-resolution CT; MAC, M. avium complex; NTM, non-tuberculous mycobacteria.
Risk factors
Are there modifiable risk factors for the development of NTM-PD in individuals with CF?
Recommendation 1: The CF Foundation and the ECFS recommend that the potential for cross-infection of NTM (particularly MABSC) between individuals with CF should be minimised by following national infection control guidelines. CF-related lung disease is a clear risk factor for the development of NTM-PD and is presumed to relate to the presence of structural lung damage, impaired mucociliary clearance and inflamed airways; all of which are thought to favour the development of chronic NTM infection.55 Cystic Fibrosis Transmembrane conductance Regulator (CFTR) dysfunction may, of itself, predispose to NTM infection (although the pathophysiology is unknown), since rates of heterozygosity for CFTR mutations within the non-CF population with pulmonary NTM disease are high (30–50%).56 57
However, other risk factors that predispose specific individuals with CF to acquire NTM or to develop NTM-PD are, for the most part, poorly understood, with many studies presenting conflicting results. Potential risk factors for NTM acquisition are listed below.
Lung function
There have been conflicting reports on whether an individual's spirometry results are related to the likelihood of finding NTM-positive samples, with some studies suggesting no association with lung function,13 a positive association of NTM acquisition with higher FEV1% predicted6 or, conversely, with worse lung function.11 15 30 Support for the possibility that NTM acquisition is more likely in CF individuals with severe lung disease comes from observations that the prevalence of NTM-positive sputum samples in patients referred for lung transplantation has been reported to be as high as 19.7%.29
Lung infection with specific pathogens
In some studies, individuals with CF with NTM-positive samples are more likely to have Staphylococcus aureus infection and less likely to have Pseudomonas aeruginosa chronic pulmonary infection.6 7 58 Other studies, however, have reported NTM positivity associated with higher rates of P. aeruginosa infection,11 and variably associated with S. maltophilia infection.6 58 In contrast, Aspergillus fumigatus has consistently been associated with the presence of NTM-positive cultures,11 15 59 with some reports indicating an association with allergic bronchopulmonary aspergillosis.7 27 60
Medications
Corticosteroids
The impact of systemic steroids on NTM acquisition is controversial. There have been suggestions that steroids may protect against58 or predispose towards NTM infection,60 or may not influence the risk of NTM acquisition.4 11 12 Recent data from non-CF populations, however, have suggested that oral as well as some types of inhaled corticosteroids are associated with increased risk of NTM acquisition.61–63
Proton pump inhibitors
The impact of proton pump inhibitor (PPI) is unclear. PPI use has been reported to be associated with the development of MAC pulmonary disease in non-CF cohorts,64 and may promote gastrointestinal survival of NTM and subsequent lung infection through gastric aspiration.
Azithromycin
Particular attention has recently been paid to the role of long-term azithromycin use as a risk factor for the acquisition of NTM. In a single centre study of CF adults, Renna et al43 reported increases in annual rates of NTM infection associated with chronic azithromycin use, postulating, through in vitro studies and mouse infection models, that azithromycin blocked autophagic killing of NTM within macrophages. While supporting findings from a previous case–control study reporting increased azithromycin use in individuals with NTM,11 other large retrospective studies have shown no such association.12 13 59 65–67 This includes a recent nested case–control analysis within the CF registry, which suggested long-term azithromycin use may protect against infection with NTM.67
Acquisition of NTM through cross-infection
Person-to-person transmission of NTM has traditionally been considered unlikely. Two separate studies have shown that patients, even siblings living in the same household for more than 10 years, have unique strains,7 68 suggesting a lack of person-to-person transmission. However, a case report from the University of Washington described a possible outbreak of M. a. massiliense in five patients48 with potential transmission occurring during synchronous clinic visits. Recently, whole genome sequencing and antimicrobial susceptibility testing performed on 168 consecutive isolates of M. abscessus from 31 patients attending an adult CF centre in the UK revealed frequent, probably indirect, transmission of M. a. massiliense between individuals with CF despite conventional cross-infection measures.69 The results of these studies indicate that cross-infection may be an important mechanism for the acquisition of M. abscessus (at least within the CF population). To date, there has been no published evidence suggesting person-to-person transmission of other NTM species.
Other factors extrapolated from data in non-CF populations or studies on M. tuberculosis that might contribute to NTM acquisition in individuals with CF include: low vitamin D,70 71 the presence of gastro-oesophageal reflux disease,64 72 low body mass index56 73 or malnutrition.74
Screening
How often should individuals with CF be screened for NTM?
Recommendation 2: The CF Foundation and the ECFS recommend that cultures for NTM be performed annually in spontaneously expectorating individuals with a stable clinical course.
Recommendation 3: The CF Foundation and the ECFS recommend that, in the absence of clinical features suggestive of NTM-PD, individuals who are not capable of spontaneously producing sputum do not require screening cultures for NTM.
Over the past two decades, a number of expert opinions and reviews have urged routine screening for NTM in the general CF population. However, the optimal frequency and methodology for NTM surveillance in individuals with CF are not known. NTM are common in the environment, and are likely to be transiently introduced on a regular basis into the airways of individuals with CF. More frequent screening will, therefore, result in detection of more positive cultures,11 many of which will not be associated with the presence of NTM-PD,6 30 58 generating anxiety in patients and caregivers and initiating further (potentially invasive) investigations. However, signs and symptoms of NTM disease are often subtle and non-specific, and the diagnosis can be delayed for years or missed altogether in the absence of effective surveillance.4 Furthermore, systematic screening may help researchers more accurately identify factors influencing poorly understood host susceptibility, acquisition, transmission and virulence of NTM. It is important to emphasise that screening refers to obtaining samples from individuals with no clinical, microbiological or radiological suspicion of NTM infection, and should be distinguished from strategies to investigate and diagnose NTM disease (covered in Diagnosis of NTM-PD in CF section).
While our understanding of those factors predisposing individuals with CF to NTM infection is incomplete, there is, nevertheless, agreement that certain patient populations are at greater risk and therefore probably require more frequent surveillance. These populations include: those with advanced lung disease and previous NTM-positive cultures, and those living in areas with high NTM prevalence. Conversely, in individuals with no recognised risk factors, the prevalence of NTM infection is likely to be low; thus less frequent, perhaps annual, surveillance is warranted. In addition, NTM screening is important before starting long-term azithromycin treatment to avoid inadvertent macrolide monotherapy in individuals with undiagnosed NTM infection (in keeping with published guidelines.75)
How should screening for NTM be performed?
Recommendation 4: The CF Foundation and the ECFS recommend that culture and smears for acid-fast bacilli (AFB) from sputum should be used for NTM screening.
Recommendation 5: The CF Foundation and the ECFS recommend against the use of oropharyngeal swabs for NTM screening.
The majority of published reports describing the prevalence of NTM in the CF population utilised AFB smear and culture from sputum as the standard screening method.4 6 7 11 13 17 To date, there has been no direct comparison between the sensitivity of samples from spontaneously expectorated sputum samples, and sputum induced by use of hypertonic saline. Analysis of induced sputum provides equal or better detection of ‘standard’ CF pathogens76 and the procedure is in widespread use to collect samples for mycobacterial culture among CF Centres worldwide. However, the Consensus Committee felt that, due to its inconvenience, induced sputum collection should not be used as a screening tool in individuals with no features suggestive of NTM-PD who are incapable of spontaneously producing sputum. As discussed in Microbiology section, there are currently no other validated screening methods to detect NTM in individuals with CF. Although positive cultures have been detected through laryngeal suction, oropharyngeal swabs, or gastric aspirate, there are insufficient data to support their use. Skin testing for delayed-type hypersensitivity against NTM antigens does not appear sufficiently sensitive or specific to use for surveillance in the CF population. Serological assays, such as IgG against Mycobacterium antigen A60 for NTM surveillance, appear promising,42 but have not been validated in the CF population.
Microbiology
What respiratory tract samples should be used to evaluate individuals with CF for suspected NTM-PD?
Recommendation 6: The CF Foundation and the ECFS recommend that culture and smears for AFB from sputum, induced sputum, bronchial washings or bronchoalveolar lavage samples can be used to evaluate individuals with CF suspected to have NTM-PD.
Recommendation 7: The CF Foundation and the ECFS recommend against the routine use of transbronchial biopsies to detect NTM in individuals with CF suspected to have NTM-PD.
Recommendation 8: The CF Foundation and the ECFS recommend against the use of oropharyngeal swabs to perform diagnostic smears and cultures in individuals with CF suspected to have NTM-PD.
Currently, sputum, induced sputum, bronchial washings and bronchoalveolar lavage samples are routinely used to evaluate individuals for suspected NTM-PD.77 Samples for NTM should be processed for smear microscopy, preferably by fluorescence, and for culture. Microscopy allows for direct evaluation of the bacterial burden, and may indicate false-negative culture results through excessive sample decontamination or overgrowth of conventional bacteria. Oropharyngeal swabs should not be used for the detection of NTM, since they do not consistently provide sufficient material for culture.77
A staged approach should be adopted for obtaining diagnostic samples; testing spontaneously expectorated or induced sputum (if available) before resorting to bronchoscopy. Although there are no published studies comparing the relative performance of these different methods for detection of NTM, the presence of negative sputum samples in individuals with radiological and clinical suspicion of NTM disease should prompt CT-guided bronchoscopic sampling, as, for example, in nodular bronchiectatic disease.78–80 While trans-bronchial biopsies can reveal NTM (on microscopy or culture) and may demonstrate granulomatous inflammation (supporting NTM disease rather than transient colonisation), they should not be obtained routinely in individuals with CF given the significant risks of bleeding and pneumothorax.81
How should respiratory tract samples from individuals with CF be cultured for NTM?
Recommendation 9: The CF Foundation and the ECFS recommend that respiratory tract samples should be cultured using both solid and liquid media.
Recommendation 10: The CF Foundation and the ECFS recommend that the incubation duration for NTM cultures should be for a minimum of 6 weeks.
Recommendation 11: The CF Foundation and the ECFS recommend that an NTM culture should be processed within 24 h of collection to optimise the detection of NTM in respiratory samples. If a delay in processing is anticipated, refrigeration of samples is advised.
The most sensitive and rapid way to detect viable mycobacteria is to culture samples (following decontamination to remove conventional bacteria and fungi) in liquid media using an automated growth detection system (such as Mycobacteria Growth Indicator Tube (MGIT)77 82 83); a process widely used around the world. However, concomitant culture on solid media may increase the diagnostic yield since NTM can be detected despite incomplete sample decontamination.84 Since decontamination procedures substantially reduce the viability of mycobacteria in samples, attempts have been made to use highly selective agar for solid culture of unprocessed sputum. A recent study, using agar designed for Burkholderia cepacia complex culture,84 demonstrated an improvement in detection of rapidly growing mycobacteria from 0.7% with conventional liquid culture to 2.8%. The duration, both of liquid and solid culture methods, has not been rigorously tested but the vast majority of pathogenic NTM will grow by 6 weeks—the current recommended duration in US and European laboratories.77
Laboratory processing of samples should ideally be performed within 24 h of collection to avoid overgrowth by conventional bacteria, which can reduce NTM viability85 and prevent successful decontamination.85 Studies have shown that refrigeration of samples may improve NTM detection from sputum samples86 and should be considered if delays longer than 24 h in processing are anticipated.
How should respiratory tract samples from individuals with CF be decontaminated to optimise the detection of NTM?
Recommendation 12: The CF Foundation and the ECFS recommend that respiratory tract samples should be decontaminated using the standard N-acetyl l-cysteine, NALC, (0.5%)-NaOH (2%) method.
Recommendation 13: The CF Foundation and the ECFS recommend that, if a sample remains contaminated with Gram-negative bacteria after standard NALC-NaOH decontamination, it should be further treated with either 5% oxalic acid or 1% chlorhexidine.
Adequate sample decontamination to remove conventional bacteria and fungi is essential to permit culture-based detection of mycobacteria,77 87 88 but often fails in CF samples given high densities of P. aeruginosa and other microbes.39–41 89 90 Since enhanced decontamination protocols adversely impact on NTM viability in samples,90 a two-step approach to sample processing should be adopted.41 Virtually all US and European clinical microbiology laboratories currently use an NALC-NaOH decontamination step prior to mycobacterial culture.41 87 88
The addition of a second decontamination step using oxalic acid has been shown to permit the recovery of NTM from persistently contaminated samples albeit with reduced sensitivity.40 Alternatively, use of 1% chlorhexidine as a first step may improve the recovery of mycobacteria, but at the expense of higher rates of residual sample contamination.89 Chlorhexidine negatively affects the performance of the MGIT automated liquid culture system, because it needs to be neutralised with lecithin; lecithin generates random fluorescence reactions from the MGIT system sensor, limiting its use.89
Should non-culture-based methods be used to detect NTM in respiratory tract samples from individuals with CF?
Recommendation 14: The CF Foundation and the ECFS recommend against the use of non-culture-based methods for detecting NTM in respiratory tract samples.
A number of studies have been published on the use of PCR-based detection methods for NTM from respiratory samples.91–95 To date, however, none have been robustly evaluated for CF sputum samples, nor have they demonstrated sufficiently high sensitivity and specificity on smear-negative samples91 to recommend their routine diagnostic use. Furthermore, the clinical significance of PCR-positive respiratory samples is currently unknown.
How should NTM isolates from individuals with CF be identified?
Recommendation 15: The CF Foundation and the ECFS recommend that all NTM isolates from individuals with CF should undergo molecular identification.
Recommendation 16: The CF Foundation and the ECFS recommend that all NTM isolates from individuals with CF should be identified to the species level, except for M. intracellulare, M. avium and M. chimaera, where identification can be limited to MAC and MABSC, which should be subspeciated.
As individual NTM species differ in their potential to cause clinical disease in humans96 and in their response to specific antibiotics, correct species identification of NTM isolates is clinically important. Moreover, in the case of M. abscessus, the ability to identify isolates to the subspecies level (M. a. abscessus, M. a. bolletii, M. a. massiliense) may predict treatment response97 and potentially permit targeted therapy.98 M. a. massiliense harbours a partial erm41 gene deletion, preventing inducible macrolide resistance,97 99 and leads to more successful outcomes with macrolide-based antibiotic regimens than in infections with M. a. abscessus (which has a full length, functional erm41 gene).97
There is no gold standard for NTM species identification. Molecular methods have now surpassed biochemical tests for NTM identification in many laboratories.100–107 Although matrix-assisted laser desorption ionisation-time of flight mass spectrometry has shown promise in providing rapid speciation of NTM,108–112 the optimal method for protein extraction from mycobacteria and the exact discriminatory power of this method have yet to be established.
Among molecular methods, three techniques are in current clinical use. The first includes line probe assays,103–105 113 which are easy to perform but costly, and permits accurate identification of the most frequently encountered NTM species but not subspeciation of M. abscessus. The second technique is PCR product restriction analysis in which amplified gene fragments are restriction digested to yield different sized fragments, which are then resolved by gel electrophoresis and correlated with specific species.114 This technique is mostly used in low-resource settings and is at least comparable to the line probe assays.106 The third technique is (partial) gene sequencing, which permits a higher level of discrimination, often to subspecies level, but is only available in laboratories with access to sequencing facilities. The choice of the optimal sequencing strategy is not straightforward. Although partial 16S ribosomal RNA (rRNA) gene sequencing provides insufficient discrimination, particularly between M. abscessus and M. chelonae,115 a number of other gene sequences (such as partial hsp65 and rpoB gene sequences) have been successfully used.107 116 For subspeciation of M. abscessus, a multilocus sequence typing approach has recently been validated.116–118 An alternative strategy close to subspeciation is to measure erm gene associated inducible macrolide resistance by phenotypic drug susceptibility testing (DST). This does not distinguish accurately between M. abscessus subspecies but does offer the data for which the subspeciation is generally performed—whether or not there is inducible macrolide resistance.
Should DST be performed on NTM isolates from individuals with CF?
Recommendation 17: The CF Foundation and the ECFS recommend that for MAC, clarithromycin susceptibility testing should be performed on an isolate recovered prior to initiation of treatment. Clarithromycin susceptibility testing should also be performed on subsequent isolates if the patient (a) fails to culture convert after 6 months of NTM treatment; (b) recultures MAC after initial culture conversion while on NTM treatment or (c) recultures MAC after completion of NTM treatment.
Recommendation 18: The CF Foundation and the ECFS recommend that for MABSC, susceptibility testing should include at least clarithromycin, cefoxitin and amikacin (and preferably also tigecycline, imipenem, minocycline, moxifloxacin and linezolid).
Recommendation 19: The CF Foundation and the ECFS recommend that DST should be performed in accordance with CLSI guidelines.
Based on current published data, the exact role of DST and its potential to guide regimen selection and predict outcomes in NTM lung disease in patients with CF, remains unknown.119 The CLSI has published guidelines on DST of NTM.17 120 121 Its European counterpart, the European Committee on Antimicrobial Susceptibility Testing (EUCAST), presently has no guidelines for DST of NTM.77
It is important to appreciate that, although CLSI guidelines provide breakpoint concentrations to interpret minimum inhibitory concentrations (MICs) as ‘susceptible’ or ‘resistant’, these cut-offs have had very limited clinical validation, and no clinical validation has been performed in patients with CF. Moreover, limited pharmacokinetic (PK) data are now available for MAC lung disease to support breakpoint concentrations,122 there are no representative PK or pharmacodynamic data to guide treatment of patients with CF.
Breakpoints for clarithromycin susceptibility of MAC have been validated in HIV-related disseminated MAC disease and in retrospective series of MAC lung disease.119 123 124 Since the presence of macrolide resistance predicts worse clinical outcomes125 126 and requires augmented treatment,126 susceptibility to macrolides should be tested on isolates prior to treatment initiation and during treatment in refractory cases defined as those individuals who (1) fail to culture convert after 6 months of NTM treatment; (2) reculture MAC after initial culture conversion while on NTM treatment or (3) reculture MAC after completion of NTM treatment.
A very recent study has shown that amikacin MICs >64 mg/L are measured only in MAC isolates that have mutations associated with amikacin resistance, that is, in the 16S rRNA gene. These strains are cultured from patients with significant aminoglycoside exposure, such as individuals with CF, and for disease caused by these strains, amikacin is unlikely to have any beneficial effect.127
For rapidly growing mycobacteria including M. abscessus, clinical validation has only been performed in series of extra-pulmonary disease,128 and only for cefoxitin, aminoglycosides and co-trimoxazole. In series of M. abscessus lung disease, the outcomes of macrolide-based treatment are generally poor and do not correlate well with in vitro susceptibilities119 129 potentially due to erm41-dependent inducible macrolide resistance and relative short duration of adequate regimens, which were often interrupted because of toxicity. Indeed, in the absence of a functional erm41 gene, response to macrolide-containing treatments has been good.94 The CLSI has recommended routine testing for inducible macrolide resistance by performing extended incubation of isolates in the presence of clarithromycin, as inducible resistance may predict treatment failure.120 For M. simiae, the role of DST is unknown, although the generally poor outcomes of treatment have been correlated with a lack of synergistic activity between rifampicin and ethambutol, an in vitro observation that still awaits clinical validation.130 Some molecular methods to assess drug susceptibility exist, but are not yet routinely available. For example, sequencing of the 16S rRNA and 23S rRNA genes can reveal mutations associated with high-level resistance to aminoglycosides and macrolides, respectively.119 127
Diagnosis of NTM-PD in CF
Should the ATS/IDSA criteria for the diagnosis of NTM-PD be used in individuals with CF?
Recommendation 20: The CF Foundation and the ECFS recommend that ATS/IDSA criteria for the diagnosis of NTM-PD should be used in individuals with CF (ATS/IDSA 2007 Statement).
Recommendation 21: The CF Foundation and the ECFS recommend that other CF pathogens and comorbidities should be considered as potential contributors to a patient's symptoms and radiological features when determining the clinical significance of NTM-positive cultures.
Recommendation 22: The CF Foundation and the ECFS recommend that NTM treatment should be considered for individuals with CF who have ATS/IDSA defined NTM-PD.
Recommendation 23: The CF Foundation and the ECFS recommend that individuals receiving azithromycin as part of their CF medical regimen who have a positive NTM culture should not continue azithromycin treatment while evaluation for NTM disease is underway, as azithromycin monotherapy may lead to resistance. A macrolide agent may be included in a multidrug treatment regimen if criteria are met for NTM disease.
In contrast to M. tuberculosis, a single positive culture of NTM does not necessarily indicate that an individual has NTM-PD. To address the difficulty of making a diagnosis of NTM-PD, the ATS/IDSA proposed a set of clinical, radiological and microbiological criteria required to define an individual as having NTM-PD (ref 22; box 1). Although these criteria have not been validated for individuals with CF, they have been widely adopted by NTM specialists around the world and provide an operational definition for NTM-PD, which supports clinical decision-making and facilitates research. The Statements Committee therefore concluded that, in the absence of an alternate, CF-validated definition, the ATS/IDSA criteria should be used for the definition of NTM-PD in individuals with CF.
Box 1. ATS/IDSA clinical and microbiologic criteria for diagnosing non-tuberculous mycobacterial pulmonary disease (NTM-PD) (based on ref 22).
Clinical (both required)
Pulmonary symptoms with nodular or cavitary opacities on chest radiograph, or a high-resolution CT scan that shows multifocal bronchiectasis with multiple small nodules.
Appropriate exclusion of other diagnoses.
Microbiologic (one of the following required)
Positive culture results from at least two expectorated sputum samples. If the results from samples are non-diagnostic, consider repeat sputum acid-fast bacilli (AFB) smears and cultures.
Positive culture results from at least one bronchial wash or lavage.
Transbronchial or other lung biopsy with mycobacterial histopathological features (granulomatous inflammation or AFB) and positive culture for NTM or biopsy showing mycobacterial histopathological features (granulomatous inflammation or AFB) and one or more sputum or bronchial washings that are culture positive for NTM.
Expert consultation should be obtained when either infrequently encountered NTM or those usually representing environmental contamination are recovered.
Patients who are suspected of having NTM-PD but who do not meet the diagnostic criteria should be followed until the diagnosis is firmly established or excluded.
Making the diagnosis of NTM-PD does not, per se, necessitate the institution of therapy, which is a decision based on potential risks and benefits of therapy for individual patients.
Microbiological criteria for NTM-PD
Individuals should have two or more positive sputum cultures of the same NTM species or one positive culture from bronchoscopic lavage or wash. The threshold for the number of positive sputum samples is derived from an observational study of individuals without CF with MAC in which 98% individuals with at least two positive sputum cultures developed progressive radiographic change compared to only 2% with one positive culture.131 The type of NTM species isolated is also important. Thus, isolation of M. abscessus is more likely to reflect NTM-PD than culturing usually non-pathogenic species such as M. gordonae and M. terrae complex.
Radiological criteria for NTM-PD
In the context of CF-related lung disease, a chest radiograph is unlikely to be of use for the investigation of NTM-PD. High-resolution CT (HRCT) scan changes supporting a diagnosis of NTM-PD would include: inflammatory nodules, new tree-in-bud opacities (particularly in areas of mild underlying bronchiectasis) and cavitation.132 However, these changes are non-specific, particularly in individuals with severe CF-related lung disease, and may reflect infection with more common CF pathogens, inadequate airway clearance or the development of allergic bronchopulmonary aspergillosis (ABPA).
Clinical criteria for NTM-PD
NTM-PD should be suspected in individuals with worsening respiratory symptoms (breathlessness, increased cough and sputum production) and/or declining pulmonary function tests that do not respond to antibiotic therapy targeting conventional CF-associated bacteria and optimised airway clearance. Night sweats, fevers, chest pains and weight loss (although uncommon) may also suggest possible NTM-PD.
NTM treatment should be considered in individuals with CF who fulfil ATS/IDSA criteria for NTM-PD. However, the decision to start treatment is a clinical one based on an amalgamation of patient factors, the NTM species involved, the risks of treatment side effects, adherence concerns and the expected outcomes of treatment.
Recommended clinical practice for diagnosis
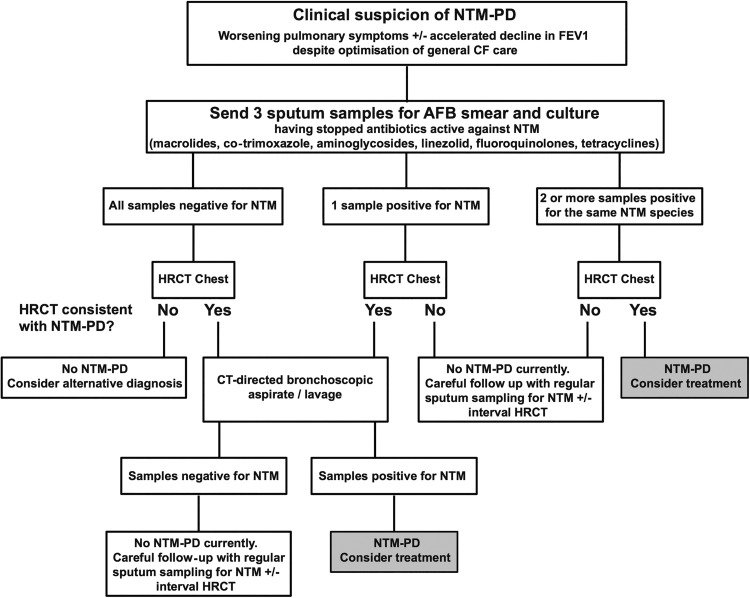
A suggested algorithm for the investigation of individuals with CF suspected of having NTM-PD is shown in figure 1.
Figure 1.
A suggested algorithm for the investigation of individuals with clinical suspicion of NTM-PD (AFB, acid-fast bacilli; CF, cystic fibrosis; FEV1, forced expiratory volume in 1 s; HRCT, high-resolution CT; NTM-PD, non-tuberculous mycobacteria pulmonary disease).
When being investigated for potential NTM-PD, individuals should discontinue drugs liable to compromise NTM culture (such as macrolides, fluoroquinolones, aminoglycosides, co-trimoxazole, linezolid and doxycycline) prior to sputum sample collection. In the case of azithromycin, intracellular accumulation within phagocytes may require a washout period of 2 weeks or more to allow for drug clearance.133 134 If sputum samples are persistently culture negative, but clinical or radiological suspicion of NTM-PD remains, bronchoscopy with targeted sampling of areas with suggestive HRCT changes may be indicated. Individuals receiving azithromycin as part of their CF medical regimen, who have a positive surveillance NTM culture, should not continue azithromycin treatment while evaluation for NTM disease is underway, as azithromycin monotherapy may lead to the development of macrolide resistance.
Other CF pathogens and comorbidities should be considered as potential contributors to a patient's symptoms and radiological features when determining the clinical significance of NTM-positive cultures. All aspects of CF care should be reviewed and optimised in order to determine the clinical significance of NTM in the sputum. Specifically, consider a trial of NTM-sparing intravenous antibiotics (ie, avoid carbapenems, cefoxitin, tigecycline, fluoroquinolones, linezolid and amikacin) that target conventional bacteria; and assess for CF-related diabetes, uncontrolled gastrointestinal reflux disease, and clinical and immunological features of ABPA. Likewise, adequate treatment of sinus disease, nutritional support and effective airway clearance strategies should be implemented.
Before starting NTM treatment, side effects, the importance of adherence to therapy and complications of treatment should be discussed with patients, and these discussions documented in the medical notes. Discussion of the risk of treatment failure should be clearly documented.
Treatment
Which antibiotic regimen should be used in individuals with CF who have ATS/IDSA-defined MABSC pulmonary disease?
Recommendation 24: The CF Foundation and the ECFS recommend that treatment of MABSC pulmonary disease should involve an intensive phase followed by a continuation phase.
Recommendation 25: The CF Foundation and the ECFS recommend that the intensive phase should include a daily oral macrolide (preferably azithromycin) in conjunction with 3–12 weeks of intravenous amikacin and one or more of the following: intravenous tigecycline, imipenem or cefoxitin, guided but not dictated by DST. The duration of intensive phase therapy should be determined by the severity of infection, the response to treatment and the tolerability of the regimen.
Recommendation 26: The CF Foundation and the ECFS recommend that the continuation phase should include a daily oral macrolide (preferably azithromycin) and inhaled amikacin, in conjunction with 2–3 of the following additional oral antibiotics: minocycline, clofazimine, moxifloxacin and linezolid, guided but not dictated by DST.
Recommendation 27: The CF Foundation and the ECFS recommend that individuals with MABSC pulmonary disease should be managed in collaboration with experts in the treatment of NTM and CF, as drug intolerance and drug-related toxicity occur frequently, and changes in antibiotic therapy are often required.
Recommendation 28: The CF Foundation and the ECFS recommend that monotherapy with a macrolide or other antimicrobial should never be used in the treatment of MABSC pulmonary disease.
There are no published randomised controlled trials evaluating treatment outcomes in individuals with M. abscessus pulmonary infections. Current treatment recommendations from the ATS and IDSA recommend consideration of a multidrug treatment regimen, but note that long-term sputum conversion is difficult to achieve and thus, alternative goals such as symptomatic improvement, radiographic regression of opacities or microbiological improvement, may be more realistic.26 The ATS/IDSA recommendations were based primarily on a single large study of 154 patients with lung disease caused by rapidly growing mycobacteria, in which more than 80% of patients were infected by M. abscessus.135 Treatment outcomes were extremely poor; however, the patients did not receive the currently recommended combination of antibiotics.
Since the publication of the last ATS/IDSA guidelines,26 there have been several studies that reported treatment outcomes in individuals without CF with pulmonary disease due to M. abscessus. Jeon et al136 described treatment outcomes in 65 non-CF adults, in South Korea, with M. abscessus lung disease, who received a standardised treatment regimen. The regimen included 4 weeks of amikacin (15 mg/kg/day in two divided doses) and cefoxitin (200 mg/kg/day in three divided doses) along with clarithromycin (1000 mg/day in two divided doses), ciprofloxacin (1000 mg/day in two divided doses) and doxycycline (200 mg/day in two divided doses). The total duration of therapy was 24 months and at least 12 months after sputum culture conversion. Fifty-four (83%) patients responded with improved symptoms and 48 (74%) with improved HRCT findings. Sputum conversion and maintenance of negative sputum cultures for more than 12 months was achieved in 38 (58%) patients. This rate was significantly lower (17%) in patients whose isolates were resistant to clarithromycin. In contrast, in the 14 (22%) patients who underwent resectional surgery, negative sputum cultures were achieved and maintained in 7 (88%) of 8 with preoperatively positive cultures. The authors concluded that a standardised regimen was moderately effective, but adverse reactions were frequent.
Among 107 patients with M. abscessus pulmonary infection at National Jewish Health in Denver, CO, 69 non-CF individuals were treated and followed for a mean duration of 34 months.129 Patients were treated with individualised treatment regimens following ATS/IDSA recommendations. Twenty (29%) patients remained culture positive, 16 (23%) converted but experienced relapse, 33 (48%) converted to negative and did not relapse, while 17 (16%) died during the study period. There were significantly more surgical patients than medical patients whose culture converted and remained negative for at least 1 year (57% vs 28%, p=0.022). As in the previous study from South Korea, surgery may have been beneficial. However, surgical management is less likely to be applicable in individuals with CF in whom focal pulmonary disease is uncommon.
In a follow-up study, Koh et al97 reported significant differences in outcomes based on which subspecies of M. abscessus was causing the infection. Treatment response rates to a standardised multidrug regimen were much higher in patients with M. a. massiliense than in those with M. a. abscessus: sputum culture conversion occurred in 88% of patients with M. a. massiliense compared with 25% with M. a. abscessus (p<0.001). All of the M. a. abscessus isolates contained a full length, functional erm41 that was shown to result in inducible macrolide resistance when the isolates were incubated with clarithromycin. In contrast, the MIC of M. a. massiliense strains did not increase after incubation with the macrolide agent because the erm41 gene contained a deletion, making it non-functional. Recent data from this same group of investigators have indicated that clarithromycin is a much stronger inducer of erm41 than azithromycin, suggesting that the latter macrolide may be a better choice when treating M. a. abscessus infections.98
Despite the clinical significance of M. abscessus lung infection in patients with CF, data on treatment outcomes are extremely limited. There is one anecdotal report that describes eradication of M. abscessus in an individual with CF who received a prolonged course of therapy with alternating month inhaled amikacin plus oral clarithromycin.137 However, this appears to be an uncommon outcome in practice. A recent case series of 52 individuals, including 15 with CF, with M. abscessus and/or M. chelonae infection, suggests that tigecycline-based regimens may be of benefit, with 10/15 individuals with CF showing some improvement.138
Recommended clinical practice for antibiotic treatment for M. abscessus pulmonary disease in CF
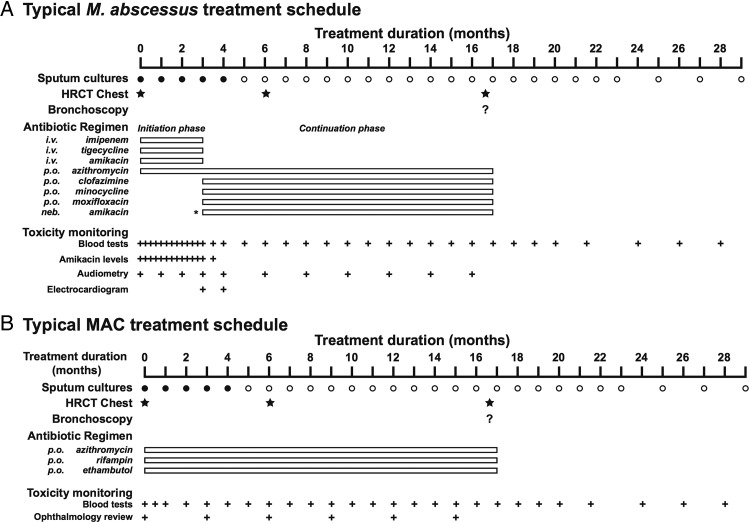
A typical treatment schedule for individuals with CF with M. abscessus infection is shown in figure 2. Antibiotic dosing regimens are listed in table 2 with important side effects/toxicities described in table 3.
Figure 2.
Typical treatment schedules for individuals with CF with Mycobacterium abscessus or MAC pulmonary disease. (A) M. abscessus treatment is divided into an initial intensive phase with an oral macrolide (preferably azithromycin) and intravenous amikacin with one or more additional intravenous antibiotics (tigecycline, imipenem, cefoxitin) for 3–12 weeks (depending on severity of infection, response to treatment, and the tolerability of the regimen), followed by a continuation phase of oral macrolide (preferably azithromycin) and inhaled amikacin with 2–3 additional antibiotics (minocycline, clofazimine, moxifloxacin, linezolid). Antibiotic choices should be guided but not dictated by drug susceptibility testing. Baseline and interval testing for drug toxicity is essential (B). MAC treatment (for clarithromycin-sensitive disease) should be with a daily oral macrolide (preferably azithromycin), rifampin and ethambutol. An initial course of injectable amikacin or streptomycin should be considered in the presence of (i) AFB smear positive respiratory tract samples, (ii) radiological evidence of lung cavitation or severe infection and (iii) systemic signs of illness. Baseline and interval testing for drug toxicity is essential (AFB, acid-fast bacilli; CF, cystic fibrosis; HRCT, high-resolution CT; MAC, Mycobacterium avium complex).
Table 2.
Antibiotic-dosing regimens used to treat Mycobacterium avium complex and Mycobacterium abscessus complex pulmonary disease in cystic fibrosis
| Antibiotic | Route | Dose suitable for children/adolescents | Dose suitable for adults |
|---|---|---|---|
| Amikacin* | Intravenous | Children: 15–30 mg/kg/dose once daily Adolescents: 10–15 mg/kg/dose once daily Maximum dose 1500 mg daily |
10–30 mg/kg once daily or 15 mg/kg/day in two divided doses Daily to 3× weekly dosing |
| Amikacin*†‡ | Nebulised | 250–500 mg/dose once or twice daily | 250–500 mg once or twice daily |
| Azithromycin | Oral | Children: 10–12 mg/kg/dose once daily Adolescents: adult dosing regimen Maximum dose 500 mg |
250–500 mg once daily |
| Cefoxitin | Intravenous | 50 mg/kg/dose thrice daily (maximum dose 12 g/day) | 200 mg/kg/day in three divided doses (maximum dose 12 g/day) |
| Clarithromycin | Oral | 7.5 mg/kg/dose twice daily (maximum dose 500 mg) | 500 mg twice daily§ |
| Clarithromycin | Intravenous | Not recommended | 500 mg twice daily§ |
| Clofazimine†¶ | Oral | 1–2 mg/kg/dose once daily (maximum dose 100 mg) | 50–100 mg once a day |
| Co-trimoxazole (sulfamethoxazole and trimethoprim) | Oral | 10–20 mg/kg/dose twice daily | 960 mg twice daily |
| Co-trimoxazole (sulfamethoxazole and Trimethoprim) | Intravenous | 10–20 mg/kg/dose twice daily | 1.44 g twice daily |
| Ethambutol | Oral | Infants and children: 15 mg/kg/dose once daily Adolescents: 15 mg/kg/dose once daily |
15 mg/kg once daily |
| Imipenem | Intravenous | 15–20 mg/kg/dose twice daily (maximum dose 1000 mg) | 1 g twice daily |
| Linezolid** | Oral | <12 years old: 10 mg/kg/dose thrice daily 12 years and older: 10 mg/kg/dose once or twice daily (maximum dose 600 mg) |
600 mg once or twice daily |
| Linezolid** | Intravenous | <12 years old: 10 mg/kg/dose thrice daily 12 years and older: 10 mg/kg/dose once or twice daily (maximum dose 600 mg) |
600 mg once or twice daily |
| Moxifloxacin | Oral | 7.5–10 mg/kg/dose once daily (maximum dose 400 mg daily) | 400 mg once daily |
| Minocycline | Oral | 2 mg/kg/dose once daily (maximum dose 200 mg) | 100 mg twice daily |
| Rifampin (Rifampicin) | Oral | 10–20 mg/kg/dose once daily (maximum dose 600 mg) | <50 kg 450 mg once daily >50 kg 600 mg once daily |
| Rifabutin | Oral | 5–10 mg/kg/dose once daily (maximum dose 300 mg) | 150–300 mg once daily 150 mg if patient taking strong CYP3A4 inhibitor 450–600 mg if patient taking strong CYP3A4 inducer |
| Streptomycin* | Intramuscular/intravenous | 20–40 mg/kg/dose once daily (maximum dose 1000 mg) | 15 mg/kg once daily (maximum dose 1000 mg) |
| Tigecycline†,†† | Intravenous | 8–11 years: 1.2 mg/kg/dose twice daily (maximum dose 50 mg) 12 years and older: 100 mg loading dose and then 50 mg once or twice daily |
100 mg loading dose and then 50 mg once or twice daily |
*Adjust dose according to levels. Usually, starting dose is 15 mg/kg aiming for a peak level of 20–30 µg/mL and trough levels of <5–10 micrograms/ml.
†As tolerated.
‡Mixed with normal saline.
§For individuals under 55 kg, many practitioners recommend 7.5 mg/kg twice daily.
¶Only available in the USA through an IND application to the FDA.
**Usually given with high dose (100 mg daily) pyridoxine (vitamin B6) to reduce risk of cytopaenias.
††Many practitioners recommend pre-dosing with one or more anti-emetics before dosing and/or gradual dose escalation from 25 mg daily to minimise nausea and vomiting.
IND, investigational new drug; FDA, Food and Drug Administration.
Table 3.
Important side effects/toxicities of antibiotics and advisable monitoring procedures for MAC and MABSC in CF
| Drug | Common side effects/toxicity | Monitoring procedures |
|---|---|---|
| Amikacin | Nephrotoxicity | Regular serum amikacin levels* Regular serum creatinine levels |
| Auditory-vestibular toxicity (tinnitus, high-frequency hearing loss) | Symptoms, baseline and interval audiograms | |
| Azithromycin | Nausea, vomiting, diarrhoea | Symptoms |
| Auditory-vestibular toxicity | Symptoms, audiogram | |
| Prolonged QT | ECG | |
| Clarithromycin | Hepatitis | Liver function tests |
| Taste disturbance | Symptoms | |
| Inhibited hepatic metabolism of rifabutin | Symptoms | |
| Cefoxitin | Fever, rash | Symptoms |
| Eosinophilia, anaemia, leucopaenia, thrombocytopaenia | Full blood count | |
| Interference with common assays to measure serum creatinine | Use alternative assay | |
| Clofazimine | Discoloration of skin† | Symptoms |
| Enteropathy (sometimes mimicking pancreatic insufficiency)† | Symptoms | |
| Nausea and vomiting | Symptoms | |
| Co-trimoxazole | Nausea, vomiting, diarrhoea | Symptoms |
| Anaemia, leucopoenia, thrombocytopaenia | Full blood count | |
| Fever, rash, Stevens-Johnson syndrome | Symptoms | |
| Ethambutol | Optic neuritis | Symptoms (loss of colour vision/acuity) Baseline and interval testing for colour vision and acuity‡ Ophthalmology opinion if symptoms occur |
| Peripheral neuropathy | Symptoms; nerve conduction studies | |
| Imipenem | Hepatitis | Liver function tests |
| Imipenem (cont) | Nausea, vomiting, diarrhoea | Symptoms |
| Linezolid | Anaemia, leucopaenia, thrombocytopaenia | Full blood count |
| Peripheral neuropathy | Symptoms/clinical evaluation/electrophysiology | |
| Optic neuritis | Symptoms (loss of colour vision/acuity) Baseline and interval testing for colour vision and acuity Ophthalmology opinion if symptoms occur |
|
| Moxifloxacin | Nausea, vomiting, diarrhoea | Symptoms |
| Insomnia, agitation, anxiety | Symptoms | |
| Tendonitis | Symptoms | |
| Photosensitivity | Symptoms | |
| Prolonged QT | ECG | |
| Minocycline | Photosensitivity | Symptoms |
| Nausea, vomiting, diarrhoea | Symptoms | |
| Vertigo | Symptoms | |
| Skin discolouration | Clinical evaluation | |
| Rifampin and rifabutin | Orange discolouration of bodily fluids (can stain contact lenses) | Symptoms |
| Hepatitis | Liver function tests | |
| Nausea, vomiting, diarrhoea | Symptoms | |
| Fever, chills | Symptoms | |
| Thrombocytopaenia | Full blood count | |
| Renal failure (rifampin) | Blood tests | |
| Increased hepatic metabolism of numerous drugs | Dose adjustment of other medications/serum levels where available | |
| Rifabutin | Leucopaenia, | Full blood count |
| Anterior uveitis (when combined with clarithromycin) | Symptoms | |
| Flu-like symptoms polyarthralgia, polymyalgia | Symptoms | |
| Streptomycin | Nephrotoxicity | Regular serum streptomycin levels Regular serum creatinine levels |
| Auditory-vestibular toxicity (tinnitus, high frequency hearing loss) | Symptoms, baseline and interval audiograms | |
| Tigecycline | Nausea, vomiting, diarrhoea | Symptoms |
| Pancreatitis | Serum amylase§ | |
| Hypoproteinaemia | Serum albumin | |
| Bilirubinaemia | Serum bilirubin |
*Usually aiming for peak levels of 20–30 µg/mL and trough levels of <5–10 µg/mL.
†It may take up to 3 months for toxicity to resolve following cessation of clofazimine due to its long half-life.
‡Monthly checks if receiving 25 mg/kg/day.
§In individuals with pancreatic sufficiency.
CF, cystic fibrosis; MABSC, Mycobacterium abscessus complex; MAC, Mycobacterium avium complex.
Given the lack of clinical trial data to inform treatment decisions there is a lot of variation in how patients are treated. An initial intensive phase is typically used to rapidly decrease the bacterial load. A combination of two intravenous drugs with demonstrated in vitro activity is administered for several weeks to months in combination with one or more oral drugs. Intravenous drug regimens of amikacin with cefoxitin and/or imipenem and/or tigecycline are the most commonly used combinations. Oral drugs with demonstrated in vitro activity include the macrolides (clarithromycin and azithromycin), linezolid, clofazimine and, occasionally, ciprofloxacin and/or moxifloxacin. After the intensive phase of therapy, patients are usually treated with at least two oral drugs in addition to a macrolide with or without inhaled antibiotics.
However, there is growing concern that treatment of M. abscessus isolates that have either a functional erm41 gene (resulting phenotypically in inducible macrolide resistance) or a 23S rRNA mutation (leading to high level constitutive macrolide resistance) may be compromised by switching from intravenous to oral therapy (given the relatively poor efficacy of oral antibiotics) and, therefore, continuous/very extended intravenous therapy with two or more effective antibiotics may be indicated in these cases.
The choice of intravenous agents is based on in vitro activity and the toxicity profile of the drug. In addition to amikacin, imipenem is perhaps the best choice as companion intravenous therapy; the drug shows in vitro activity and the side effect profile is better than that of cefoxitin and tigecycline. In the study reported by Jeon et al,136 60% of the patients started on cefoxitin had to have the drug discontinued due to drug-related toxicity, after a median of 22 days of treatment. Neutropaenia occurred in 51% and thrombocytopaenia in 6% of patients on cefoxitin. Tigecycline has a low MIC against M. abscessus and showed efficacy against M. abscessus in combination.138 However, it is associated with significant nausea and vomiting, which has made it difficult to administer for a prolonged period.138
There are few oral drugs with significant in vitro activity against M. abscessus; the macrolides are the only oral drugs with consistent activity although their use may be potentially limited by inducible resistance (as described above) or acquired point mutations in the 23S rRNA. There are no clinical trials comparing azithromycin to clarithromycin in M. abscessus infection, so the choice of which macrolide to use is typically based on the in vitro activity, side effects profile and consideration of drug interactions. Clarithromycin has slightly better in vitro activity than azithromycin but there are conflicting reports regarding the impact of erm41 gene expression with each of these drugs.98 139 140 Clarithromycin is a stronger inhibitor of the P450 enzyme system than is azithromycin, so drug interactions are more common.
Linezolid shows in vitro activity in approximately 50% of M. abscessus isolates (although there is considerable geographical variation); however, haematological (anaemia, thrombocytopaenia) and neurological (peripheral neuropathy, optic neuritis) toxicities are common, particularly when linezolid is dosed 600 mg two times a day for prolonged courses. For this reason, many practitioners give 600 mg once daily to reduce the risk of adverse effects. However, care should be exercised in individuals chronically co-infected with methicillin-resistant Staphylococcus aureus (MRSA) since long-term linezolid therapy may encourage MRSA resistance. The fluoroquinolones and minocycline/doxycycline rarely show in vitro activity although they were included in the standardised treatment regimen used in the report by Jeon et al.98 Finally, clofazimine has significant in vitro activity against M. abscessus.141 However, this drug, used to treat leprosy, is not readily available in the USA at this time, although it can be obtained with an IRB-approved protocol through submission of an individual patient use IND to the Food and Drug Administration. Instructions for this process can be found on the NTM Info and Research, Inc, website (http://www.ntminfo.org/clofazimine).
The lack of oral antibiotics with activity against M. abscessus has led clinicians to use inhaled amikacin, usually during the continuation phase of therapy. There are no studies correlating treatment outcomes in patients with M. abscessus infection with the dose of inhaled amikacin and, therefore, there is a great deal of variation in the dose used (250–500 mg), and frequency of administration (daily to twice daily). A recent study, targeting treatment refractory NTM patients, most of whom were without CF with M. abscessus, evaluated the effect of adding inhaled amikacin to their oral and/or intravenous drug regimens.142 Among the 20 patients with persistently positive cultures, 8 (40%) had at least one negative culture and 5 (25%) had persistently negative cultures after addition of inhaled amikacin. Inhaled amikacin was stopped in 7 (35%) due to toxicity. There is currently significant interest in the potential use of a liposomal formulation of amikacin (which may improve drug delivery within the lung and into infected macrophages) as part of a multidrug regimen for both M. abscessus and MAC. Large multicentre studies are ongoing.
The optimum duration of therapy is not known. Based on studies in individuals without CF, even prolonged treatment regimens were associated with high rates of failure and recurrence. Many patients who do not convert their cultures to negative on therapy may still benefit from continuing or repeating courses of treatment.
Treatment for MAC
Which antibiotic regimen should be used in individuals with CF who have ATS/IDSA-defined MAC pulmonary disease?
Recommendation 29: The CF Foundation and the ECFS recommend the same antibiotic regimen for treatment of all species within the MAC.
Recommendation 30: The CF Foundation and the ECFS recommend that clarithromycin-sensitive MAC pulmonary disease should be treated with a daily oral antibiotic regimen containing a macrolide (preferably azithromycin), rifampin and ethambutol.
Recommendation 31: The CF Foundation and the ECFS recommend against the use of intermittent (three times per week) oral antibiotic therapy to treat MAC pulmonary disease.
Recommendation 32: The CF Foundation and the ECFS recommend that monotherapy with a macrolide or other antimicrobial agent should never be used in the treatment of MAC pulmonary disease.
Recommendation 33: The CF Foundation and the ECFS recommend that an initial course of intravenous amikacin should be considered for the treatment of MAC pulmonary disease in the presence of one or more of the following: (i) AFB smear positive respiratory tract samples, (ii) radiological evidence of lung cavitation or severe infection and (iii) systemic signs of illness.
Recommendation 34: The CF Foundation and the ECFS recommend that clarithromycin-resistant MAC pulmonary disease should be managed in collaboration with experts in the treatment of NTM and CF.
There are very few published randomised controlled trials evaluating treatment for MAC pulmonary disease (MAC-PD) in non-HIV-positive patients and none in individuals with CF. In the pre-macrolide era, a UK trial of individuals without CF and with largely cavitary disease reported that those randomised to receive rifampin and ethambutol had a combined failure/relapse rate of 41% compared to 16% of patients randomised to receive rifampin, ethambutol and isoniazid (p=0.033).143 In a subsequent study on a similar cohort, patients randomised to receive rifampin, ethambutol and clarithromycin had an all-cause mortality of 48% compared to 30% of patients randomised to receive rifampin, ethambutol and ciprofloxacin.144 However, only 13% of patients in the clarithromycin group failed treatment or relapsed compared to 23% in the ciprofloxacin group.
In addition, there have been several non-comparator studies evaluating outcomes in HIV-negative patients with MAC-PD. The majority utilised a three oral drug regimen including a macrolide (clarithromycin or azithromycin), a rifamycin (rifampin or rifabutin) and ethambutol, in combination with an initial course of an aminoglycoside (streptomycin, amikacin or kanamycin).124 125 145–148 The culture conversion rate varied considerably between studies (13–82%), but on the whole, in 55–65% of patients, the culture converted after 6–12 months treatment and, when reported, the mean time from starting treatment to culture conversion was 3–5 months.124 125 Treatment failure was associated with previous MAC-PD treatment, cavitary disease, smear positivity, clarithromycin resistance at initiation of treatment, intolerance of NTM therapy and acquired clarithromycin resistance.124 125 145 147–149
An alternative regimen using clofazimine with a macrolide and ethambutol in a study of 30 patients resulted in a culture conversion rate of 87% and a treatment success rate of 67%.150 Although 5 (19%) patients relapsed an average of 17 months after completing treatment, all MAC isolates remained clarithromycin sensitive, raising the possibility of reinfection rather than treatment failure.151 In another case series utilising clofazimine in combination with clarithromycin and minocycline, the culture conversion rate was 64% in patients completing the study (47% overall), which may indicate the importance of ethambutol as part of the multidrug regimen in the treatment of MAC-PD.152
Clarithromycin resistance developed in up to 15% of patients receiving treatment for MAC-PD and this was generally associated with clarithromycin monotherapy or the prescription of inadequate companion medications.124–126 146–149 When taken in combination with ethambutol and a rifamycin, acquired clarithromycin resistance developed in only 12/303 (4%) of patients. In the context of clarithromycin resistance, the best treatment responses were seen in patients who underwent surgical resection and received >6 months of an injectable aminoglycoside (amikacin or streptomycin),124–126 as 11/14 (79%) so treated achieved culture conversion compared to 1/27 (4%) of those not surgically resected and not receiving injectables.123
While intermittent and daily dosing regimens appear equally effective in several case series of individuals without CF, intermittent regimens may be associated with less toxicity, better tolerability and adherence, and lower cost.147 148 However, a large multicentre study utilising an intermittent dosing regimen in individuals with moderate or severe MAC-PD (including many with cavitary disease and with prior treatment failure), reported a culture conversion rate of only 13% after 12 months of treatment.146 151 There have, to date, been no studies in individuals with CF to determine an optimal dosing regimen for MAC therapy, but concerns about drug absorption and lung penetration in CF have meant that many centres have adopted daily dosing protocols.
It is unclear if use of an aminoglycoside during the initial phase of MAC antibiotic therapy is beneficial. In a multicentre study involving 146 HIV-negative patients with MAC-PD, participants were randomised to receive intramuscular streptomycin (15 mg/kg) or placebo thrice weekly for the first 3 months of therapy, in addition to clarithromycin, rifampin and ethambutol. Streptomycin-treated patients had a significantly higher culture conversion rate after approximately 2 years of treatment than did placebo patients (71% vs 51%, p<0.05), but a third of patients in each group experienced sputum relapse and there were no significant differences in symptoms or radiological response between groups.153 Furthermore, there was no statistically significant difference in culture conversion rates between individuals who received an initial course of intramuscular kanamycin (78%) compared to those who did not (58%) in a non-randomised study involving patients with MAC-PD.125 Recently, the use of aerosolised amikacin in addition to standard multidrug macrolide-based regimens was reported in six HIV-negative individuals with MAC-PD who had failed standard therapy.154 While four patients were culture negative after 6 months of therapy, one later cultured M. chelonae (resistant to amikacin), two re-cultured MAC and one patient was unable to tolerate prolonged therapy with aerosolised amikacin. A recent case series of the impact of nebulised amikacin (250 mg once or twice daily)142 in 20 individuals without CF with treatment refractory NTM-PD (of whom 5 had MAC) reported adverse events in 35% of cases. Two patients discontinued therapy due to hearing loss. Studies examining the use of liposomal amikacin (which may have a better side effects profile) for the treatment of NTM in individuals with CF are ongoing.
Recommended clinical practice antibiotic treatment for MAC-PD in CF
A typical treatment schedule for individuals with CF with MAC infection is shown in figure 2. Antibiotic dosing regimens are listed in table 2 with important side effects/toxicities described in table 3.
Individuals with clarithromycin sensitive MAC-PD should be treated with a daily oral antibiotic regimen that includes a macrolide, rifampin and ethambutol (15 mg/kg), consistent with the ATS/IDSA recommendations for individuals with severe nodular bronchiectatic disease.26 Intermittent oral antibiotic therapy is not recommended due to the nature of the underlying lung disease and concerns regarding antibiotic absorption in CF. While there are no head to head trials showing a difference in outcome between individuals with MAC-PD treated with clarithromycin or azithromycin, the latter may be the macrolide of choice in CF, as it can be taken once daily, its serum levels may be less affected by rifamycins122 and it has well established benefits in individuals with CF in addition to its effects on NTM.
Individuals with a high bacterial load (suggested by smear positivity, radiological evidence of lung cavitation and/or significant inflammatory change or the presence of systemic symptoms) may benefit from an initial (1–3 month) course of injectable amikacin or streptomycin, in addition to the standard three-drug regimen for MAC-PD. While the available data do not show a difference in toxicity between amikacin regimens dosed at 15 mg/kg once daily or 25 mg/kg thrice weekly, ototoxicity was found in 37% of all participants (associated with older age and larger cumulative dose), vestibular toxicity in 8% (usually reversible) and nephrotoxicity in 15% (usually mild and reversible).155 Streptomycin, although less widely used for MAC-PD than amikacin, may have less ototoxicity than amikacin.155 The use of aerosolised amikacin in place of an intravenous aminoglycoside may be preferable in terms of reduced burden of care and toxicity, but outcome data are limited and it is unlikely to be helpful for patients with cavitary disease in whom drug levels at the site of infection may be subtherapeutic.
The major risk factors for the development of clarithromycin-resistant MAC-PD are macrolide monotherapy and prior macrolide treatment with inadequate companion medications. Thus, macrolides (often prescribed for their anti-inflammatory effects in CF) should be discontinued immediately following isolation of a mycobacterial species, and macrolides should never be prescribed in the treatment of MAC-PD without two appropriate companion antibiotics.
Macrolide therapy is not generally recommended in the context of clarithromycin-resistant MAC-PD,26 but macrolides may still be beneficial in this context in CF due to their non-antibiotic properties. Individuals with clarithromycin-resistant MAC-PD may respond to a regimen including a parenteral aminoglycoside, a rifamycin (usually rifabutin) and ethambutol, in addition to one or more companion medications (accepting that there are limited data to guide practice)26 126 144 such as a quinolone or clofazimine. Rifabutin may be useful in the treatment of clarithromycin-resistant MAC-PD, but adverse events (particularly blood dyscrasias, gastrointestinal upset and polyarthralgia) are more common and often necessitate dose reduction or complete cessation of treatment.156–158 Surgical resection might also be helpful in selected individuals with localised severe bronchiectatic disease, but this management is less likely to be useful in CF as MAC-PD is more likely to be diffuse.
Ethambutol ocular toxicity (optic or retrobulbar neuritis) may present with blurred vision, decreased acuity, central scotomas, impaired red-green colour discrimination and peripheral visual field defects. Ocular toxicity was identified in 6% of individuals without CF with MAC-PD receiving ethambutol at a dose of 25 mg/kg/day for the first 2 months followed by 15 mg/kg/day for the remainder of treatment.159 Ocular toxicity is more likely to occur in the context of MAC-PD than in patients receiving tuberculosis (TB) treatment due to the longer duration of therapy. While individuals prescribed ethambutol should have regular visual acuity and colour vision testing, visual symptoms often occur before measurable changes can be identified. Thus, patients should be educated about the potential side effects of ethambutol and encouraged to self-report changes in vision, following which ethambutol therapy should be discontinued until an ophthalmological assessment has taken place.
It is not uncommon for more than one NTM species to be isolated from an individual with CF.6 30 In these circumstances, continued microbiological surveillance is advisable to determine which species is/are persistently positive and which is/are likely to be causing disease. NTM-PD is also commonly associated with ABPA and/or the identification of Aspergillus spp in sputum or lavage specimens. As rifamycins increase the hepatic metabolism of azole antifungal agents, the treatment of Aspergillus in the context of MAC-PD is more difficult. One approach is to use rifabutin in place of rifampin (as it is the rifamycin with the least cytochrome P450 enzyme induction) in conjunction with the usual companion medications for MAC and voriconazole, or posaconazole, which may be less affected by rifabutin co-medication than voriconazole is, with adjustment of drug doses according to levels.160 161 If therapeutic drug monitoring (TDM) is not available, other approaches include using nebulised amikacin or clofazimine in place of rifampin.150
Treatment: generic recommendations
What outcome monitoring should be performed in individuals with CF receiving treatment for NTM-PD?
Recommendation 35: The CF Foundation and the ECFS recommend that individuals with CF receiving NTM treatment should have expectorated or induced sputum samples sent for NTM culture every 4–8 weeks throughout the entire course of treatment to assess the microbiological response.
Recommendation 36: The CF Foundation and the ECFS recommend that a schedule for detecting drug toxicity (including hearing loss, visual loss, renal impairment and liver function test abnormalities) should be set in place at the time of NTM treatment initiation and implemented throughout treatment based on the specific drugs prescribed.
Recommendation 37: The CF Foundation and the ECFS recommend that an HRCT scan of the lungs should be performed shortly before starting NTM treatment and at the end of NTM treatment, to assess the radiological response.
What duration of antibiotic therapy is recommended for individuals with CF receiving treatment for NTM-PD?
Recommendation 38: The CF Foundation and the ECFS recommend that NTM antibiotic therapy should be prescribed for 12 months beyond culture conversion (defined as three consecutive negative cultures, with the time of conversion being the date of the first of the three negative cultures) as long as no positive cultures are obtained during those 12 months.
Recommendation 39: The CF Foundation and the ECFS recommend that individuals who fail to culture convert despite optimal NTM therapy may benefit from long-term suppressive antibiotic treatment.
Treatment: TDM
Should TDM be performed in individuals with CF receiving treatment for NTM-PD?
Recommendation 40: The CF Foundation and the ECFS recommend that, when amikacin is given intravenously or when streptomycin is given intravenously or intramuscularly, serum levels should be monitored and dosing adjusted to minimise ototoxicity and nephrotoxicity.
Recommendation 41: The CF Foundation and the ECFS recommend against routinely obtaining serum levels of other anti-mycobacterial drugs. However, absorption of oral medications is often reduced in CF. Therefore use of TDM should be considered for individuals failing to improve despite taking recommended drug regimens or for those on concomitant medications with significant interactions with NTM drugs.
TDM seeks to quantify the relationship between drug dose, serum (plasma) concentration and clinical response,162 and to thereby maximise therapeutic response while avoiding toxicity. The potential benefits of TDM during NTM treatment in individuals with CF include adjusting drug dosing to:
Correct for drug–drug interactions that could adversely affect serum antibiotic levels: Drug–drug interactions frequently occur among agents used to treat NTM. Rifampin (more than rifabutin) may increase the metabolism of several drugs including clarithromycin, azithromycin and moxifloxacin, while rifabutin increases azithromycin levels and decreases moxifloxacin levels.122 163
Maximise the PK and pharmacodynamic (PD) parameters of antibiotics to optimise efficacy: The PK/PD indices that correlate with clinical efficacy vary by antimicrobial agent.122 164 165 To exert maximal activity, drugs such as aminoglycosides and ethambutol require high peak concentrations relative to the pathogen's minimal inhibitory concentration (Cmax/MIC). Ciprofloxacin and rifampin require a high concentration time or area under the plasma concentration curve measured over 24 h to MIC ratio (AUC0–24/MIC) and β-lactam agents require as much time as possible whereby the concentration persists above the infecting organism's MIC (%T> MIC). Macrolide agents such as azithromycin have weak concentration-dependent effects and time effects, but these agents exert their activity through intracellular activity, tissue penetration and prolonged, persistent effects, due to their long half-life.166
Overcome CF-related differences in absorption, distribution and clearance of drugs: Individuals with CF have different renal and non-renal clearance of several drugs when compared to individuals without CF, due to reduced bioavailability, increased volume of distribution and more rapid clearance. In addition, hepatic disease and diabetes may further influence drug metabolism and absorption. Several recent reviews have addressed evidence-based dosing for various agents used for treatment of pulmonary exacerbations in CF.167–170 While the relevance of the recommended dosing schedules is unknown for treatment of NTM, it is possible that individuals with CF would need higher dosages of mycobacterial drugs.
With the exception of aminoglycosides, the clinical utility of TDM during treatment for NTM is unknown for individuals with and without CF due to a lack of rigorous studies, although some experts have recommended TDM for mycobacterial agents on a case-by-case basis.165 A recent retrospective study assessed the PK and pharmacodynamic parameters for 481 patients with disease caused by MAC.122 Peak serum concentrations within reference/normal ranges were only achieved for ethambutol, clarithromycin and azithromycin, in 52%, 44% and 65% of patients, respectively. In addition, pharmacodynamic targets for Cmax/MIC or AUC0–24/MIC were rarely achieved. However, these observations were not linked with clinical outcomes.
Another recent evaluation of the potential utility for TDM in 130 individuals without CF treated for MAC found no association between peak plasma/MIC ratios for clarithromycin, rifampin or ethambutol, and clinical outcomes.171 As previously observed, rifampin had a substantial impact on clarithromycin levels; those treated with both drugs had a median peak plasma clarithromycin concentration of 0.3 µg/mL, while those treated with rifabutin had a median peak plasma concentration of 1.8 µg/mL, and those with M. abscessus (n=60) treated without rifampin had a median peak plasma concentration of 3.8 µg/mL. In all, 97% of patients with MAC treated with daily therapy and 100% of patients on intermittent therapy reached the target of 2 µg/mL for clarithromycin. These experts concluded that TDM for treatment of MAC lung disease may not be beneficial (although the effects of dose optimisation on clinical outcomes were not evaluated).
To the best of our knowledge, there is only one case series, published over a decade ago, examining the potential role of TDM in CF. Ten patients with CF with mycobacterial disease (6 with MAC, 3 with M. abscessus and 1 with M. tuberculosis) had serum drug concentration measurements performed 2 and 4 h after ingestion.164 Monitoring serum levels at two time points helped distinguish between poor absorption and delayed absorption. Half of the patients had inadequate serum levels for one or more drugs, and one patient clinically improved following dose adjustments that achieved target serum levels. However, target concentrations were not achieved for several patients. Notably, this study did not compare outcomes in patients with and without TDM.
Treatment: adjuvant therapy and surgery
In the context of infectious disease, adjuvants have been defined as ‘therapies that act by rendering the organism more susceptible to attack by antibiotics or the host immune system, by rendering the organism less virulent or killing it by other means’.172 A number of approaches have been proposed as candidates for adjuvant therapy in NTM infection in CF, including interferon γ (IFNγ; or agents that promote IFNγ release) and vitamin D. Drug delivery vehicles, such as liposomes, may be considered adjuvants. Liposomes have been studied as a mode of delivering amikacin for infection with P. aeruginosa in CF,173 and this approach is also being evaluated (clinicaltrials.gov/show/NCT01315236) for NTM.
Does IFNγ therapy improve treatment outcomes in individuals with CF who have NTM-PD?
Recommendation 42: The CF Foundation and the ECFS recommend against the use of IFNγ as adjuvant therapy for NTM-PD in individuals with CF.
IFNγ plays a critical role in the host defence against NTM infection: (1) deficiencies in IFNγ signalling (caused by deleterious mutations174 or neutralising autoantibodies175) lead to (usually disseminated) NTM infection in individuals without CF; (2) inoculation of mice deficient in IFNγ or IFNγ receptors results in disseminated NTM infection;176 (3) addition of IFNγ to NTM-infected human macrophages in vitro enhances intracellular killing probably through autophagy stimulation.43
In non-CF individuals, adjuvant IFNγ therapy in NTM infection has been examined in several studies.177 178 An uncontrolled trial of IFNγ was conducted in seven patients with presumed primary immunodeficiency (three with familial susceptibility to MAC and four with idiopathic CD4 lymphopaenia) who had disseminated NTM disease refractory to conventional antibiotic therapy.177 All the patients improved with the introduction of subcutaneous IFNγ two or three times per week.
In a randomised, placebo controlled trial, 32 patients with pulmonary NTM disease (30 with MAC) were randomised to receive either intramuscular IFNγ (1×106 IU) or placebo once daily for 4 weeks and then three times weekly for 20 weeks178 in addition to daily oral azithromycin, ciprofloxacin, ethambutol and rifampin. The primary outcome (a composite end point of improvements in symptoms, radiology and microbiology) was achieved at 6 months by 72% (13/18) of patients in the IFNγ arm compared to 36% (5/14) receiving placebo (p=0.037). The greater response rate with IFNγ was sustained at 12 months after completion of treatment. However, the small study size, the use of composite end points and the lack of microbiological response after 6 months treatment mean that these data need to be interpreted with caution.
Furthermore three large trials (ClinicalTrials.gov Identifiers NCT00001318, NCT00111397 and NCT00043355) examining IFNγ therapy for pulmonary NTM disease remain unpublished or have been terminated (potentially due to lack of efficacy), again questioning the role of IFNγ adjuvant therapy.
Does vitamin D supplementation improve treatment outcomes in individuals with CF who have NTM-PD?
Recommendation 43: The CF Foundation and the ECFS recommend that vitamin D should be supplemented according to national CF care guidelines.
Vitamin D is thought to play a critical role in host defence against mycobacteria. In vitro and ex vivo treatment with vitamin D of human macrophages infected with M. tuberculosis enhances intracellular killing (through stimulating antimicrobial peptide production179 and autophagy.180) Furthermore, several epidemiological studies have shown an association of vitamin D deficiency with reactivation of TB181 182 and, recently, the presence of NTM-PD.71 However, interventional trials of vitamin D supplementation in patients with active pulmonary TB have had mixed results,183 and there are no trials of vitamin D as an adjuvant treatment for NTM disease.
Should surgery be considered in individuals with CF who have NTM-PD?
Recommendation 44: The CF Foundation and the ECFS recommend that lung resection should only be considered under extraordinary circumstances and in consultation with experts on the treatment of NTM and CF.
Surgical resection has been used extensively in the management of pulmonary mycobacterial infection in order to excise localised infection, debulk severe disease, or excise cavities or damaged lung into which antibiotic penetration may be impaired. In no cases has surgery been used as a substitute for antibiotic therapy. There are no randomised trials of surgery for the treatment of pulmonary NTM disease in any patient group. While many publications report the use of lung resection (pneumonectomy, lobectomy or segmentectomy) with combination antibiotic therapy in NTM infection, most are case reports with no comparator group receiving only medical therapy thereby preventing objective assessment of the efficacy of surgery.
Nonetheless, three series of individuals without CF do contain some comparison data although the potential for selection bias of patients considered suitable for surgery makes interpretation difficult. The first series136 comprised of 65 patients, from South Korea, with pulmonary M. abscessus infection. Surgery was performed in 14 patients who failed to achieve sputum culture conversion, became culture positive again after a period of culture negativity or experienced disease-related complications such as haemoptysis. Of the eight patients who were sputum culture positive before surgery, seven became culture negative postoperatively (compared to culture conversion rates of 38/65 for the group as a whole). A second study, from the USA,129 reported outcomes for 69 patients with pulmonary M. abscessus infection all treated with combination antibiotics, 23 of whom underwent additional surgical resection. Indications for surgery included the presence of localised bronchiectasis, cavitary disease and haemoptysis. In the surgical group, significantly more patients (13/23) became persistently sputum culture negative compared to those in the medical treatment only group (13/46). A third study, also from the USA, described outcomes in 51 patients with macrolide-resistant MAC-PD.126 Individuals receiving both surgical resection and injectable aminoglycoside therapy had greater sputum conversion rates (11/14 patients) than those receiving neither treatment modality (2/37 patients).
A recent review of case series published over the last 40 years184 suggests that localised resection (lobectomy or segmentectomy) should be considered for severe, localised, unilateral NTM disease that has failed to respond to conventional antibiotic therapy. In the context of CF, however, localised NTM disease is extremely rare (or at least very difficult to identify), and the risks of thoracic surgery are high and therefore the potential benefits of surgical resection limited.
Transplantation
Should individuals with CF with current or previous NTM-positive cultures be referred for lung transplantation?
Recommendation 45: The CF Foundation and the ECFS recommend that all individuals with CF being considered for lung transplantation should be evaluated for NTM-PD.
Recommendation 46: The CF Foundation and the ECFS recommend that the presence of current or previous respiratory tract samples positive for NTM should not preclude individuals being considered for lung transplantation.
Recommendation 47: The CF Foundation and the ECFS recommend that individuals with CF who have NTM-PD and are being evaluated for transplantation should start treatment prior to transplant listing.
Recommendation 48: The CF Foundation and the ECFS recommend that individuals with CF receiving NTM treatment with sequential negative cultures may be eligible for transplant listing.
Recommendation 49: The CF Foundation and the ECFS recommend that individuals with CF who have completed treatment for NTM-PD with apparent eradication of the organism may be eligible for transplant listing.
Recommendation 50: The CF Foundation and the ECFS recommend that the presence of persistent MABSC or MAC infection despite optimal therapy is not an absolute contraindication to lung transplant referral.
The International Society for Heart and Lung Transplantation (ISHLT) International Guidelines lists ‘colonisation with highly resistant or virulent mycobacteria’ as a relative contra-indication for selection as a lung transplant candidate.185 There is, however, limited published information on transplant outcomes for individuals with previous or concurrent NTM infection, with very few reports (usually from single centres) specifically examining CF cohorts.186 187
The risk of NTM infection post-transplantation is not well defined. A study of 201 CF and non-CF transplant recipients188 suggested that postoperative NTM acquisition was associated with increased mortality (HR 2.61), independent of bronchiolitis obliterans syndrome. However, these data should be interpreted keeping the following in mind: very little data were available on the presence of pulmonary NTM pretransplant; the vast majority of patients did not have CF or even bronchiectasis; and non-NTM-related causes were major contributors to death in fatal cases. In contrast, a recent study of CF and non-CF transplant recipients189 reported that 53 of 237 individuals (22.4%) acquired NTM-positive cultures postoperatively (70% MAC, 10% MABSC), of whom two fulfilled ATS/IDSA criteria for NTM-PD. Although overall mortality was not affected by NTM acquisition, four patients developed persistent surgical site infection (three with M. abscessus), of whom one died of disseminated NTM infection. The potential for M. abscessus to cause significant postoperative complications is supported by a review of outcomes from 31 transplant centres190 indicating frequent soft tissue and surgical site infections, and two deaths, attributable to M. abscessus infection.
The largest CF-specific case series comes from the University of North Carolina Chapel Hill experience between 1990 and 2003.29 One hundred and forty-six patients with CF underwent lung transplantation and 31 listed for transplantation. Of those individuals referred, 19.7% were NTM culture positive pretransplant. Rates of NTM following lung transplantation were 3.4%. Pretransplant infection with M. abscessus was recognised as a significant risk factor for recurrence of NTM post-transplantation. Although there was no effect on mortality, post-transplant NTM infection caused significant morbidity as patients developed M. abscessus-associated skin and soft tissue infection or pulmonary disease caused by MAC and other NTM species. There are several published case series of successful outcomes for individuals with CF who have culture positive M. abscessus infection at the time of transplantation.186 However, NTM-related complications in this group may be more frequent, and include persistent soft tissue or wound infections,186 empyema and disseminated NTM infection.164 191 Although a small series, the University of North Carolina (UNC) report suggests no effect of the presence of pretransplant M. abscessus positive cultures on post-transplant mortality.187
The Consensus statements Committee concluded that all individuals with CF should be evaluated for NTM disease prior to referral for lung transplantation, given the very high reported rates of NTM culture positivity for this group, and the fact that untreated NTM infection may represent an increased (and potentially modifiable) postoperative risk. Consequently, if NTM-PD is diagnosed, treatment should be started prior to transplant listing.
Conclusion
The management of individuals with CF infected with NTM is extremely challenging. The limited amounts of published research and clinical trial data provide inadequate evidence to base management decisions on how best to screen, diagnose, detect and treat NTM-PD. As a response to this urgent clinical need, the CF Foundation and the ECFS formed a committee of clinicians, scientists and infectious disease experts to develop recommendations to guide and assist clinicians in the management of NTM-PD in individuals with CF. The committee believes these recommendations should serve as a benchmark for current medical care while providing a framework to inform the development of clinical, translation and basic research studies to generate robust evidence on which to base future iterations of these management guidelines, leading to better outcomes for individuals with CF infected with NTM.
Supplementary Material
Acknowledgments
The authors would like to thank David Young, Chair of the CFF Pharmacist Mentorship Committee, for review of treatment regimens and drug tables.
Footnotes
Contributors: All the authors contributed to the expert committee on guidelines development (Co-chairs: RAF, CSH; Steering committee: RAF, CSH, BCM, KNO, LS, KAS, SEH; Committee subgroups: Epidemiology and Risk Factors (KNO (lead), IS-G, KLW): Screening (JAN (lead), RLG, KK; Microbiology (J-LH (lead), RJW, JvI, RAF); Treatment (CLD (lead), DB, LS, ARS, CSH); and Transplantation (PGN (lead), PC).
Funding: Cystic Fibrosis Foundation; European Cystic Fibrosis Society, The Wellcome Trust and Cambridge NIHR BRC (RAF); Intramural programme of the National Heart, Lung, and Blood Institute, NIH (KNO); Vaincre La Mucoviscidose (VLMIC1014 and RF20120600689) and the Région Ile-de-France Domaine d'Intérêt Majeur Maladies Infectieuses et Emergentes (J-LH); CF Foundation Clinical Research Award (NICK13A0) (JAN); Imperial College London NIHR Respiratory BRU (DB).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Smith MJ, Efthimiou J, Hodson ME, et al. . Mycobacterial isolations in young adults with cystic fibrosis. Thorax 1984;39:369–75. 10.1136/thx.39.5.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hjelte L, Petrini B, Kallenius G, et al. . Prospective study of mycobacterial infections in patients with cystic fibrosis. Thorax 1990;45:397–400. 10.1136/thx.45.5.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilby JM, Gilligan PH, Yankaskas JR, et al. . Nontuberculous mycobacteria in adult patients with cystic fibrosis. Chest J 1992;102:70–5. 10.1378/chest.102.1.70 [DOI] [PubMed] [Google Scholar]
- 4.Aitken ML, Burke W, McDonald G, et al. . Nontuberculous mycobacterial disease in adult cystic fibrosis patients. Chest J 1993;103:1096–9. 10.1378/chest.103.4.1096 [DOI] [PubMed] [Google Scholar]
- 5.Hjelt K, Hojlyng N, Howitz P, et al. . The role of mycobacteria other than tuberculosis (Mott) in patients with cystic-fibrosis. Scand J Infect Dis 1994;26:569–76. 10.3109/00365549409011815 [DOI] [PubMed] [Google Scholar]
- 6.Olivier KN, Weber DJ, Wallace RJ Jr, et al. . Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med 2003;167:828–34. 10.1164/rccm.200207-678OC [DOI] [PubMed] [Google Scholar]
- 7.Sermet-Gaudelus I, Le Bourgeois M, Pierre-Audigier C, et al. . Mycobacterium abscessus and children with cystic fibrosis. Emerg Infect Dis 2003;9:1587–91. 10.3201/eid0912.020774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitritz L, Griese M, Roggenkamp A, et al. . Prospective study on nontuberculous mycobacteria in patients with and without cystic fibrosis. Med Microbiol Immunol 2004;193:209–17. 10.1007/s00430-003-0195-9 [DOI] [PubMed] [Google Scholar]
- 9.Rodman DM, Polis JM, Heltshe SL, et al. . Late diagnosis defines a unique population of long-term survivors of cystic fibrosis. Am J Respir Crit Care Med 2005;171:621–6. 10.1164/rccm.200403-404OC [DOI] [PubMed] [Google Scholar]
- 10.Valenza G, Tappe D, Turnwald D, et al. . Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J Cyst Fibros 2008;7:123–7. 10.1016/j.jcf.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 11.Levy I, Grisaru-Soen G, Lerner-Geva L, et al. . Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg Infect Dis 2008;14:378–84. 10.3201/eid1403.061405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girón RM, Máiz L, Barrio I, et al. . Nontuberculous mycobacterial infection in patients with cystic fibrosis: a multicenter prevalence study. Arch Bronconeumol 2008;44:679–84. 10.1016/S0300-2896(08)75777-6 [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan DK, Yau Y, Corey M, et al. . Non-tuberculous mycobacteria in children with cystic fibrosis: isolation, prevalence, and predictors. Pediatr Pulmonol 2009;44:1100–6. 10.1002/ppul.21106 [DOI] [PubMed] [Google Scholar]
- 14.Razvi S, Saiman L. Nontuberculous mycobacteria in cystic fibrosis. Pediatr Infect Dis J 2007;26:263–4. [DOI] [PubMed] [Google Scholar]
- 15.Esther CR, Esserman DA, Gilligan P, et al. . Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 2010;9:117–23. 10.1016/j.jcf.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martiniano SL, Sontag MK, Nick JA, et al. . Nontuberculous mycobacterial infection in cystic fibrosis patients at the Colorado Cf Center. Pediatr Pulmonol 2011;304. [Google Scholar]
- 17.Roux A-L, Catherinot E, Ripoll F, et al. . Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol 2009;47:4124–8. 10.1128/JCM.01257-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salsgiver EL, Fink AK, Knapp EA, et al. . Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. 10.1378/chest.15-0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adjemian J, Olivier KN, Prevots DR. Nontuberculous mycobacteria among patients with cystic fibrosis in the United States: screening practices and environmental risk. Am J Respir Crit Care Med 2014;190:581–6. 10.1164/rccm.201405-0884OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adékambi T, Berger P, Raoult D, et al. . rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int J Syst Evol Microbiol 2006;56(Pt 1):133–43. 10.1099/ijs.0.63969-0 [DOI] [PubMed] [Google Scholar]
- 21.Bange FC, Kirschner P, Böttger EC. Recovery of mycobacteria from patients with cystic fibrosis. J Clin Microbiol 1999;37:3761–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adékambi T, Reynaud-Gaubert M, Greub G, et al. . Amoebal coculture of “Mycobacterium massiliense” sp. nov. from the sputum of a patient with hemoptoic pneumonia. J Clin Microbiol 2004;42:5493–501. 10.1128/JCM.42.12.5493-5501.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry Annual Data Report 2010. 2010. http://www.cysticfibrosisdata.org/LiteratureRetrieve.aspx?ID=132651
- 24.Qvist T, Gilljam M, Jönsson B, et al. . Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J Cyst Fibros 2015;14:46–52. 10.1016/j.jcf.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry Annual Data Report 2013. 2013. https://www.cff.org/2013_CFF_Patient_Registry_Annual_Data_Report.pdf
- 26.Griffith DE, Aksamit T, Brown-Elliott BA, et al. . An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 27.Catherinot E, Roux AL, Vibet MA, et al. . Mycobacterium avium and Mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J Cyst Fibros 2013;12:74–80. 10.1016/j.jcf.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 28.Qvist T, Pressler T, Høiby N, et al. . Shifting paradigms of nontuberculous mycobacteria in cystic fibrosis. Respir Res 2014;15:41 10.1186/1465-9921-15-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalermskulrat W, Sood N, Neuringer IP, et al. . Non-tuberculous mycobacteria in end stage cystic fibrosis: implications for lung transplantation. Thorax 2006;61:507–13. 10.1136/thx.2005.049247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martiniano SL, Sontag MK, Daley CL, et al. . Clinical significance of a first positive nontuberculous mycobacteria culture in cystic fibrosis. Ann Am Thorac Soc 2014;11:36–44. 10.1513/AnnalsATS.201309-310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou MP, Clements AC, Thomson RM. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect Dis 2014;14:279 10.1186/1471-2334-14-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh WJ, Chang B, Jeong BH, et al. . Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis (Seoul) 2013;75:199–204. 10.4046/trd.2013.75.5.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andréjak C, Thomsen VO, Johansen IS, et al. . Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med 2010;181:514–21. 10.1164/rccm.200905-0778OC [DOI] [PubMed] [Google Scholar]
- 34.Prevots DR, Shaw PA, Strickland D, et al. . Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010;182:970–6. 10.1164/rccm.201002-0310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis 2010;16:1576–83. 10.3201/eid1610.091201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons S, van Ingen J, Hsueh PR, et al. . Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis 2011;17:343–9. 10.3201/eid1703.100604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adjemian J, Olivier KN, Seitz AE, et al. . Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med 2012;186:553–8. 10.1164/rccm.201205-0913OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marras TK, Mendelson D, Marchand-Austin A, et al. . Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerg Infect Dis 2013;19:1889–91. 10.3201/eid1911.130737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whittier S, Hopfer RL, Knowles MR, et al. . Improved recovery of mycobacteria from respiratory secretions of patients with cystic fibrosis. J Clin Microbiol 1993;31:861–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittier S, Olivier K, Gilligan P, et al. . Proficiency testing of clinical microbiology laboratories using modified decontamination procedures for detection of nontuberculous mycobacteria in sputum samples from cystic fibrosis patients. The Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. J Clin Microbiol 1997;35:2706–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bange FC, Böttger EC. Improved decontamination method for recovering mycobacteria from patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis 2002;21:546–8. 10.1007/s10096-002-0760-y [DOI] [PubMed] [Google Scholar]
- 42.Ferroni A, Sermet-Gaudelus I, Le Bourgeois M, et al. . Measurement of immunoglobulin G against mycobacterial antigen A60 in patients with cystic fibrosis and lung infection due to Mycobacterium abscessus. Clin Infect Dis 2005;40:58–66. 10.1086/426442 [DOI] [PubMed] [Google Scholar]
- 43.Renna M, Schaffner C, Brown K, et al. . Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest 2011;121:3554–63. 10.1172/JCI46095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med 2007;176:306–13. 10.1164/rccm.200702-201OC [DOI] [PubMed] [Google Scholar]
- 45.Falkinham JO., III Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis 2011;17:419–24. 10.3201/eid1703.101510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomson R, Tolson C, Carter R, et al. . Isolation of nontuberculous mycobacteria (NTM) from household water and shower aerosols in patients with pulmonary disease caused by NTM. J Clin Microbiol 2013;51:3006–11. 10.1128/JCM.00899-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feazel LM, Baumgartner LK, Peterson KL, et al. . Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci USA 2009;106:16393–9. 10.1073/pnas.0908446106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aitken ML, Limaye A, Pottinger P, et al. . Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 2012;185:231–2. 10.1164/ajrccm.185.2.231 [DOI] [PubMed] [Google Scholar]
- 49.Bryant JM, Grogono DM, Parkhill J, et al. . Transmission of M abscessus in patients with cystic fibrosis—authors’ reply. Lancet 2013;382:504 10.1016/S0140-6736(13)61709-2 [DOI] [PubMed] [Google Scholar]
- 50.Tomashefski JF Jr, Stern RC, Demko CA, et al. . Nontuberculous mycobacteria in cystic fibrosis. An autopsy study. Am J Respir Crit Care Med 1996;154(2 Pt 1):523–8. 10.1164/ajrccm.154.2.8756832 [DOI] [PubMed] [Google Scholar]
- 51.O'Connell ML, Birkenkamp KE, Kleiner DE, et al. . Lung manifestations in an autopsy-based series of pulmonary or disseminated nontuberculous mycobacterial disease. Chest 2012;141:1203–9. 10.1378/chest.11-0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waters V, Ratjen F. Antibiotic treatment for nontuberculous mycobacteria lung infection in people with cystic fibrosis. Cochrane Database Syst Rev 2014;12:CD010004 10.1002/14651858.CD010004.pub3 [DOI] [PubMed] [Google Scholar]
- 53.Waters V, Ratjen F. Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis. Cochrane Database Syst Rev 2012;11:CD009528 10.1002/14651858.CD009528.pub2 [DOI] [PubMed] [Google Scholar]
- 54.Robinson KA, Saldanha IJ, McKoy NA. Development of a framework to identify research gaps from systematic reviews. J Clin Epidemiol 2011;64:1325–30. 10.1016/j.jclinepi.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 55.Sexton P, Harrison AC. Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J 2008;31:1322–33. 10.1183/09031936.00140007 [DOI] [PubMed] [Google Scholar]
- 56.Kim RD, Greenberg DE, Ehrmantraut ME, et al. . Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066–74. 10.1164/rccm.200805-686OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ziedalski TM, Kao PN, Henig NR, et al. . Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest 2006;130:995–1002. 10.1378/chest.130.4.995 [DOI] [PubMed] [Google Scholar]
- 58.Olivier KN, Weber DJ, Lee JH, et al. . Nontuberculous mycobacteria. II: nested-cohort study of impact on cystic fibrosis lung disease. Am J Respir Crit Care Med 2003;167:835–40. 10.1164/rccm.200207-679OC [DOI] [PubMed] [Google Scholar]
- 59.Verregghen M, Heijerman HG, Reijers M, et al. . Risk factors for Mycobacterium abscessus infection in cystic fibrosis patients; a case-control study. J Cyst Fibros 2012;11:340–3. 10.1016/j.jcf.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 60.Mussaffi H, Rivlin J, Shalit I, et al. . Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. Eur Respir J 2005;25:324–8. 10.1183/09031936.05.00058604 [DOI] [PubMed] [Google Scholar]
- 61.Andréjak C, Nielsen R, Thomsen VØ, et al. . Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 2013;68:256–62. 10.1136/thoraxjnl-2012-201772 [DOI] [PubMed] [Google Scholar]
- 62.Hojo M, Iikura M, Hirano S, et al. . Increased risk of nontuberculous mycobacterial infection in asthmatic patients using long-term inhaled corticosteroid therapy. Respirology 2012;17:185–90. 10.1111/j.1440-1843.2011.02076.x [DOI] [PubMed] [Google Scholar]
- 63.Dirac MA, Horan KL, Doody DR, et al. . Environment or host? A case-control study of risk factors for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012;186:684–91. 10.1164/rccm.201205-0825OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomson RM, Armstrong JG, Looke DF. Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest 2007;131:1166–72. 10.1378/chest.06-1906 [DOI] [PubMed] [Google Scholar]
- 65.Coolen N, Morand P, Martin C, et al. . Reduced risk of nontuberculous mycobacteria in cystic fibrosis adults receiving long-term azithromycin. J Cyst Fibros 2015;14:594–9. [DOI] [PubMed] [Google Scholar]
- 66.Catherinot E, Roux AL, Vibet MA, et al. . Inhaled therapies, azithromycin and Mycobacterium abscessus in cystic fibrosis patients. Eur Respir J 2013;41:1101–6. 10.1183/09031936.00065612 [DOI] [PubMed] [Google Scholar]
- 67.Binder AM, Adjemian J, Olivier KN, et al. . Epidemiology of nontuberculous mycobacterial infections and associated chronic macrolide use among persons with cystic fibrosis. Am J Respir Crit Care Med 2013;188:807–12. 10.1164/rccm.201307-1200OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bange FC, Brown BA, Smaczny C, et al. . Lack of transmission of mycobacterium abscessus among patients with cystic fibrosis attending a single clinic. Clin Infect Dis 2001;32:1648–50. 10.1086/320525 [DOI] [PubMed] [Google Scholar]
- 69.Bryant JM, Grogono DM, Greaves D, et al. . Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 2013;381:1551–60. 10.1016/S0140-6736(13)60632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gelder CM, Hart KW, Williams OM, et al. . Vitamin D receptor gene polymorphisms and susceptibility to Mycobacterium malmoense pulmonary disease. J Infect Dis 2000;181:2099–102. 10.1086/315489 [DOI] [PubMed] [Google Scholar]
- 71.Jeon K, Kim SY, Jeong BH, et al. . Severe vitamin D deficiency is associated with non-tuberculous mycobacterial lung disease: a case-control study. Respirology 2013;18:983–8. 10.1111/resp.12109 [DOI] [PubMed] [Google Scholar]
- 72.Koh WJ, Lee JH, Kwon YS, et al. . Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest 2007;131:1825–30. 10.1378/chest.06-2280 [DOI] [PubMed] [Google Scholar]
- 73.Okumura M, Iwai K, Ogata H, et al. . Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Intern Med 2008;47:1465–72. 10.2169/internalmedicine.47.1114 [DOI] [PubMed] [Google Scholar]
- 74.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004;8:286–98. [PubMed] [Google Scholar]
- 75.Mogayzel PJ Jr, Naureckas ET, Robinson KA, et al. . Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2013;187:680–9. 10.1164/rccm.201207-1160OE [DOI] [PubMed] [Google Scholar]
- 76.Al-Saleh S, Dell SD, Grasemann H, et al. . Sputum induction in routine clinical care of children with cystic fibrosis. J Pediatr 2010;157:1006–11.e1. 10.1016/j.jpeds.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 77. Delogu G, Herrmann JL. Mycobacterium species. In: Cornaglia G, Courcol R, Herrmann J-L, Kahlmeter G, Peigue-Lafeuille H, Vila J, eds. European Manual of Clinical Microbiology. Paris: Société Française de Microbiologie (SFM). 2012:297–307. [Google Scholar]
- 78.Tanaka E, Amitani R, Niimi A, et al. . Yield of computed tomography and bronchoscopy for the diagnosis of Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med 1997;155:2041–6. 10.1164/ajrccm.155.6.9196113 [DOI] [PubMed] [Google Scholar]
- 79.Sugihara E, Hirota N, Niizeki T, et al. . Usefulness of bronchial lavage for the diagnosis of pulmonary disease caused by Mycobacterium avium-intracellulare complex (MAC) infection. J Infect Chemother 2003;9:328–32. 10.1007/s10156-003-0267-1 [DOI] [PubMed] [Google Scholar]
- 80.van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med 2013;34:103–9. 10.1055/s-0033-1333569 [DOI] [PubMed] [Google Scholar]
- 81.Tukey MH, Wiener RS. Population-based estimates of transbronchial lung biopsy utilization and complications. Respir Med 2012;106:1559–65. 10.1016/j.rmed.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pfyffer GE, Welscher HM, Kissling P, et al. . Comparison of the Mycobacteria Growth Indicator Tube (MGIT) with radiometric and solid culture for recovery of acid-fast bacilli. J Clin Microbiol 1997;35:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leitritz L, Schubert S, Bücherl B, et al. . Evaluation of BACTEC MGIT 960 and BACTEC 460TB systems for recovery of mycobacteria from clinical specimens of a university hospital with low incidence of tuberculosis. J Clin Microbiol 2001;39:3764–7. 10.1128/JCM.39.10.3764-3767.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esther CR Jr, Hoberman S, Fine J, et al. . Detection of rapidly growing mycobacteria in routine cultures of samples from patients with cystic fibrosis. J Clin Microbiol 2011;49:1421–5. 10.1128/JCM.02379-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Traore I, Slosarek M. Survival of mycobacteria in sputum at different temperatures. Czech Med 1981;4:203–8. [PubMed] [Google Scholar]
- 86.Tessema B, Beer J, Emmrich F, et al. . Rate of recovery of Mycobacterium tuberculosis from frozen acid-fast-bacillus smear-positive sputum samples subjected to long-term storage in Northwest Ethiopia. J Clin Microbiol 2011;49:2557–61. 10.1128/JCM.00059-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steingart KR, Ng V, Henry M, et al. . Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 2006;6:664–74. 10.1016/S1473-3099(06)70602-8 [DOI] [PubMed] [Google Scholar]
- 88.Brown-Elliott BA, Griffith DE, Wallace RJ Jr. Diagnosis of nontuberculous mycobacterial infections. Clin Lab Med 2002;22:911–25, vi 10.1016/S0272-2712(02)00018-5 [DOI] [PubMed] [Google Scholar]
- 89.Ferroni A, Vu-Thien H, Lanotte P, et al. . Value of the chlorhexidine decontamination method for recovery of nontuberculous mycobacteria from sputum samples of patients with cystic fibrosis. J Clin Microbiol 2006;44:2237–9. 10.1128/JCM.00285-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buijtels PC, Petit PL. Comparison of NaOH-N-acetyl cysteine and sulfuric acid decontamination methods for recovery of mycobacteria from clinical specimens. J Microbiol Methods 2005;62:83–8. 10.1016/j.mimet.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 91.Peter-Getzlaff S, Lüthy J, Böddinghaus B, et al. . Development and evaluation of a molecular assay for detection of nontuberculous mycobacteria by use of the cobas amplicor platform. J Clin Microbiol 2008;46:4023–8. 10.1128/JCM.01101-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Franco-Alvarez de Luna F, Ruiz P, Gutiérrez J, et al. . Evaluation of the GenoType Mycobacteria Direct assay for detection of Mycobacterium tuberculosis complex and four atypical mycobacterial species in clinical samples. J Clin Microbiol 2006;44:3025–7. 10.1128/JCM.00068-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seagar AL, Prendergast C, Emmanuel FX, et al. . Evaluation of the GenoType Mycobacteria Direct assay for the simultaneous detection of the Mycobacterium tuberculosis complex and four atypical mycobacterial species in smear-positive respiratory specimens. J Med Microbiol 2008;57(Pt 5):605–11. 10.1099/jmm.0.47484-0 [DOI] [PubMed] [Google Scholar]
- 94.Emler S, Feldmann K, Giacuzzo V, et al. . Multicenter evaluation of a pathogenic mycobacterium screening probe. J Clin Microbiol 2001;39:2687–9. 10.1128/JCM.39.7.2687-2689.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Devine M, Moore JE, Xu J, et al. . Detection of mycobacterial DNA from sputum of patients with cystic fibrosis. Ir J Med Sci 2004;173:96–8. 10.1007/BF02914566 [DOI] [PubMed] [Google Scholar]
- 96.Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 2002;15:716–46. 10.1128/CMR.15.4.716-746.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koh WJ, Jeon K, Lee NY, et al. . Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 2011;183:405–10. 10.1164/rccm.201003-0395OC [DOI] [PubMed] [Google Scholar]
- 98.Choi GE, Shin SJ, Won CJ, et al. . Macrolide treatment for Mycobacterium abscessus and Mycobacterium massillense infection and inducible resistance. Am J Respir Crit Care Med 2012;186:917–25. 10.1164/rccm.201111-2005OC [DOI] [PubMed] [Google Scholar]
- 99.Kim HY, Kim BJ, Kook Y, et al. . Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol Immunol 2010;54:347–53. 10.1111/j.1348-0421.2010.00221.x [DOI] [PubMed] [Google Scholar]
- 100.Couto I, Machado D, Viveiros M, et al. . Identification of nontuberculous mycobacteria in clinical samples using molecular methods: a 3-year study. Clin Microbiol Infect 2010;16:1161–4. 10.1111/j.1469-0691.2009.03076.x [DOI] [PubMed] [Google Scholar]
- 101.Padilla E, González V, Manterola JM, et al. . Comparative evaluation of the new version of the INNO-LiPA Mycobacteria and genotype Mycobacterium assays for identification of Mycobacterium species from MB/BacT liquid cultures artificially inoculated with Mycobacterial strains. J Clin Microbiol 2004;42:3083–8. 10.1128/JCM.42.7.3083-3088.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tortoli E, Nanetti A, Piersimoni C, et al. . Performance assessment of new multiplex probe assay for identification of mycobacteria. J Clin Microbiol 2001;39: 1079–84. 10.1128/JCM.39.3.1079-1084.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tortoli E, Mariottini A, Mazzarelli G. Evaluation of INNO-LiPA MYCOBACTERIA v2: improved reverse hybridization multiple DNA probe assay for mycobacterial identification. J Clin Microbiol 2003;41:4418–20. 10.1128/JCM.41.9.4418-4420.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lebrun L, Weill FX, Lafendi L, et al. . Use of the INNO-LiPA-MYCOBACTERIA assay (version 2) for identification of Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex isolates. J Clin Microbiol 2005;43:2567–74. 10.1128/JCM.43.6.2567-2574.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suffys PN, da Silva Rocha A, de Oliveira M, et al. . Rapid identification of Mycobacteria to the species level using INNO-LiPA Mycobacteria, a reverse hybridization assay. J Clin Microbiol 2001;39:4477–82. 10.1128/JCM.39.12.4477-4482.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Devallois A, Goh KS, Rastogi N. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J Clin Microbiol 1997;35:2969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ringuet H, Akoua-Koffi C, Honore S, et al. . hsp65 sequencing for identification of rapidly growing mycobacteria. J Clin Microbiol 1999;37:852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saleeb PG, Drake SK, Murray PR, et al. . Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2011;49:1790–4. 10.1128/JCM.02135-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lotz A, Ferroni A, Beretti JL, et al. . Rapid identification of mycobacterial whole cells in solid and liquid culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2010;48:4481–6. 10.1128/JCM.01397-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pignone M, Greth KM, Cooper J, et al. . Identification of mycobacteria by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. J Clin Microbiol 2006;44:1963–70. 10.1128/JCM.01959-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.El Khéchine A, Couderc C, Flaudrops C, et al. . Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of mycobacteria in routine clinical practice. PLoS ONE 2011;6:e24720 10.1371/journal.pone.0024720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bille E, Dauphin B, Leto J, et al. . MALDI-TOF MS Andromas strategy for the routine identification of bacteria, mycobacteria, yeasts, Aspergillus spp. and positive blood cultures. Clin Microbiol Infect 2012;18:1117–25. 10.1111/j.1469-0691.2011.03688.x [DOI] [PubMed] [Google Scholar]
- 113.Lebrun L, Gonullu N, Boutros N, et al. . Use of INNO-LIPA assay for rapid identification of mycobacteria. Diagn Microbiol Infect Dis 2003;46:151–3. 10.1016/S0732-8893(03)00042-7 [DOI] [PubMed] [Google Scholar]
- 114.Telenti A, Marchesi F, Balz M, et al. . Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol 1993;31:175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leao SC, Tortoli E, Euzéby JP, et al. . Proposal that Mycobacterium massiliense and Mycobacterium bolletii be united and reclassified as Mycobacterium abscessus subsp. bolletii comb. nov., designation of Mycobacterium abscessus subsp. abscessus subsp. nov. and emended description of Mycobacterium abscessus. Int J Syst Evol Microbiol 2011;61(Pt 9):2311–13. 10.1099/ijs.0.023770-0 [DOI] [PubMed] [Google Scholar]
- 116.Macheras E, Roux AL, Ripoll F, et al. . Inaccuracy of single-target sequencing for discriminating species of the Mycobacterium abscessus group. J Clin Microbiol 2009;47:2596–600. 10.1128/JCM.00037-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Macheras E, Roux AL, Bastian S, et al. . Multilocus sequence analysis and rpoB sequencing of Mycobacterium abscessus (sensu lato) strains. J Clin Microbiol 2011;49:491–9. 10.1128/JCM.01274-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Macheras E, Konjek J, Roux AL, et al. . Multilocus sequence typing scheme for the Mycobacterium abscessus complex. Res Microbiol 2014;165:82–90. 10.1016/j.resmic.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 119.van Ingen J, Boeree MJ, van Soolingen D, et al. . Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Update 2012;15:149–61. 10.1016/j.drup.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 120.Clinical and Laboratory Standards Institute. M24-A2 Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes’ approved standard. 31. 2nd edn Wayne, PA: Clinical and Laboratory Standards Institute, 2011. [PubMed] [Google Scholar]
- 121.Roux AL, Catherinot E, Ripoll F, et al. . Multicenter Study of Prevalence of Nontuberculous Mycobacteria in Patients with Cystic Fibrosis in France. J Clin Microbiol 2009;47:4124–8. 10.1128/JCM.01257-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Ingen J, Egelund EF, Levin A, et al. . The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med 2012;186:559–65. 10.1164/rccm.201204-0682OC [DOI] [PubMed] [Google Scholar]
- 123.Chaisson RE, Benson CA, Dube MP, et al. . Clarithromycin therapy for bacteremic mycobacterium-avium complex disease—a randomized, double-blind, dose-ranging study in patients with AIDS. Ann Intern Med 1994;121:905–11. 10.7326/0003-4819-121-12-199412150-00001 [DOI] [PubMed] [Google Scholar]
- 124.Wallace RJ, Brown BA, Griffith DE, et al. . Clarithromycin regimens for pulmonary Mycobacterium avium complex—the first 50 patients. Am J Respir Crit Care Med 1996;153:1766–72. 10.1164/ajrccm.153.6.8665032 [DOI] [PubMed] [Google Scholar]
- 125.Tanaka E, Kimoto T, Tsuyuguchi K, et al. . Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med 1999;160:866–72. 10.1164/ajrccm.160.3.9811086 [DOI] [PubMed] [Google Scholar]
- 126.Griffith DE, Brown-Elliott BA, Langsjoen B, et al. . Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2006;174:928–34. 10.1164/rccm.200603-450OC [DOI] [PubMed] [Google Scholar]
- 127.Brown-Elliott BA, Iakhiaeva E, Griffith DE, et al. . In vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates. J Clin Microbiol 2013;51:3389–94. 10.1128/JCM.01612-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wallace RJ, Swenson JM, Silcox VA, et al. . Treatment of nonpulmonary infections due to mycobacterium-fortuitum and mycobacterium-chelonei on the basis of invitro susceptibilities. J Infect Dis 1985;152:500–14. 10.1093/infdis/152.3.500 [DOI] [PubMed] [Google Scholar]
- 129.Jarand J, Levin A, Zhang LN, et al. . Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 2011;52:565–71. 10.1093/cid/ciq237 [DOI] [PubMed] [Google Scholar]
- 130.van Ingen J, Totten SE, Heifets LB, et al. . Drug susceptibility testing and pharmacokinetics question current treatment regimens in Mycobacterium simiae complex disease. Int J Antimicrob Agents 2012;39:173–6. 10.1016/j.ijantimicag.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 131.Tsukamura M. Diagnosis of disease caused by mycobacterium-avium complex. Chest 1991;99:667–9. 10.1378/chest.99.3.667 [DOI] [PubMed] [Google Scholar]
- 132.Kim JS, Tanaka N, Newell JD, et al. . Nontuberculous mycobacterial infection: CT scan findings, genotype, and treatment responsiveness. Chest 2005;128:3863–9. 10.1378/chest.128.6.3863 [DOI] [PubMed] [Google Scholar]
- 133.Wilms EB, Touw DJ, Heijerman HG, et al. . Azithromycin maintenance therapy in patients with cystic fibrosis: a dose advice based on a review of pharmacokinetics, efficacy, and side effects. Pediatr Pulmonol 2012;47:658–65. 10.1002/ppul.21620 [DOI] [PubMed] [Google Scholar]
- 134.Wilms EB, Touw DJ, Heijerman HG. Pharmacokinetics of azithromycin in plasma, blood, polymorphonuclear neutrophils and sputum during long-term therapy in patients with cystic fibrosis. Ther Drug Monit 2006;28:219–25. 10.1097/01.ftd.0000195617.69721.a5 [DOI] [PubMed] [Google Scholar]
- 135.Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis 1993;147:1271–8. 10.1164/ajrccm/147.5.1271 [DOI] [PubMed] [Google Scholar]
- 136.Jeon K, Kwon OJ, Lee NY, et al. . Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 2009;180:896–902. 10.1164/rccm.200905-0704OC [DOI] [PubMed] [Google Scholar]
- 137.Colin AA, Ali-Dinar T. Aerosolized amikacin and oral clarithromycin to eradicate Mycobacterium abscessus in a patient with cystic fibrosis: an 8-year follow-up. Pediatr Pulmonol 2010;45:626–7. 10.1002/ppul.21222 [DOI] [PubMed] [Google Scholar]
- 138.Wallace RJ Jr, Dukart G, Brown-Elliott BA, et al. . Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J Antimicrob Chemother 2014;69:1945–53. 10.1093/jac/dku062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maurer FP, Castelberg C, Quiblier C, et al. . Erm(41)-dependent inducible resistance to azithromycin and clarithromycin in clinical isolates of Mycobacterium abscessus. J Antimicrob Chemother 2014;69:1559–63. 10.1093/jac/dku007 [DOI] [PubMed] [Google Scholar]
- 140.Maurer FP, Bruderer VL, Ritter C, et al. . Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob Agents Chemother 2014;58:3828–36. 10.1128/AAC.02448-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Ingen J, Totten SE, Helstrom NK, et al. . In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother 2012;56:6324–7. 10.1128/AAC.01505-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Olivier KN, Shaw PA, Glaser TS, et al. . Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc 2014;11:30–5. 10.1513/AnnalsATS.201307-231OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Research Committee of the British Thoracic Society. First randomised trial of treatments for pulmonary disease caused by M avium intracellulare, M malmoense, and M xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol. Thorax 2001;56:167–72. 10.1136/thorax.56.3.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jenkins PA, Campbell IA, Banks J, et al. . Clarithromycin vs ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax 2008;63:627–34. 10.1136/thx.2007.087999 [DOI] [PubMed] [Google Scholar]
- 145.Kobashi Y, Matsushima T. The effect of combined therapy according to the guidelines for the treatment of Mycobacterium avium complex pulmonary disease. Intern Med 2003;42:670–5. 10.2169/internalmedicine.42.670 [DOI] [PubMed] [Google Scholar]
- 146.Lam PK, Griffith DE, Aksamit TR, et al. . Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2006;173:1283–9. 10.1164/rccm.200509-1531OC [DOI] [PubMed] [Google Scholar]
- 147.Griffith DE, Brown BA, Cegielski P, et al. . Early results (at 6 months) with intermittent clarithromycin-including regimens for lung disease due to Mycobacterium avium complex. Clin Infect Dis 2000;30:288–92. 10.1086/313644 [DOI] [PubMed] [Google Scholar]
- 148.Griffith DE, Brown BA, Girard WM, et al. . Azithromycin-containing regimens for treatment of Mycobacterium avium complex lung disease. Clin Infect Dis 2001;32:1547–53. 10.1086/320512 [DOI] [PubMed] [Google Scholar]
- 149.Dautzenberg B, Piperno D, Diot P, et al. . Clarithromycin in the treatment of Mycobacterium avium lung infections in patients without AIDS. Clarithromycin Study Group of France. Chest J 1995;107:1035–40. 10.1378/chest.107.4.1035 [DOI] [PubMed] [Google Scholar]
- 150.Field SK, Cowie RL. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest 2003;124:1482–6. 10.1378/chest.124.4.1482 [DOI] [PubMed] [Google Scholar]
- 151.Wallace RJ Jr, Brown-Elliott BA, McNulty S, et al. . Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest 2014;146:276–82. 10.1378/chest.13-2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Roussel G, Igual J. Clarithromycin with minocycline and clofazimine for Mycobacterium avium intracellulare complex lung disease in patients without the acquired immune deficiency syndrome. GETIM. Groupe d'Etude et de Traitement des Infections a Mycobacteries. Int J Tuberc Lung Dis 1998;2: 462–70. [PubMed] [Google Scholar]
- 153.Kobashi Y, Matsushima T, Oka M. A double-blind randomized study of aminoglycoside infusion with combined therapy for pulmonary Mycobacterium avium complex disease. Respir Med 2007;101:130–8. 10.1016/j.rmed.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 154.Davis KK, Kao PN, Jacobs SS, et al. . Aerosolized amikacin for treatment of pulmonary Mycobacterium avium infections: an observational case series. BMC Pulm Med 2007;7:2 10.1186/1471-2466-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Peloquin CA, Berning SE, Nitta AT, et al. . Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis 2004;38:1538–44. 10.1086/420742 [DOI] [PubMed] [Google Scholar]
- 156.Griffith DE, Brown BA, Girard WM, et al. . Adverse events associated with high-dose rifabutin in macrolide-containing regimens for the treatment of Mycobacterium avium complex lung disease. Clin Infect Dis 1995;21: 594–8. 10.1093/clinids/21.3.594 [DOI] [PubMed] [Google Scholar]
- 157.Griffith DE, Brown BA, Girard WM, et al. . Azithromycin activity against Mycobacterium avium complex lung disease in patients who were not infected with human immunodeficiency virus. Clin Infect Dis 1996;23:983–9. 10.1093/clinids/23.5.983 [DOI] [PubMed] [Google Scholar]
- 158.Griffith DE, Brown BA, Wallace RJ Jr. Varying dosages of rifabutin affect white blood cell and platelet counts in human immunodeficiency virus—negative patients who are receiving multidrug regimens for pulmonary Mycobacterium avium complex disease. Clin Infect Dis 1996;23:1321–2. 10.1093/clinids/23.6.1321 [DOI] [PubMed] [Google Scholar]
- 159.Griffith DE, Brown-Elliott BA, Shepherd S, et al. . Ethambutol ocular toxicity in treatment regimens for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2005;172:250–3. 10.1164/rccm.200407-863OC [DOI] [PubMed] [Google Scholar]
- 160.Schwiesow JN, Iseman MD, Peloquin CA. Concomitant use of voriconazole and rifabutin in a patient with multiple infections. Pharmacotherapy 2008;28:1076–80. 10.1592/phco.28.8.1076 [DOI] [PubMed] [Google Scholar]
- 161.Krishna G, Parsons A, Kantesaria B, et al. . Evaluation of the pharmacokinetics of posaconazole and rifabutin following co-administration to healthy men. Curr Med Res Opin 2007;23:545–52. 10.1185/030079906X167507 [DOI] [PubMed] [Google Scholar]
- 162.Yew WW. Therapeutic drug monitoring in antituberculosis chemotherapy. Ther Drug Monit 1998;20:469–72. 10.1097/00007691-199810000-00004 [DOI] [PubMed] [Google Scholar]
- 163.Wallace RJ Jr, Brown BA, Griffith DE, et al. . Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. intracellulare infection. J Infect Dis 1995;171:747–50. 10.1093/infdis/171.3.747 [DOI] [PubMed] [Google Scholar]
- 164.Gilljam M, Berning SE, Peloquin CA, et al. . Therapeutic drug monitoring in patients with cystic fibrosis and mycobacterial disease. Eur Respir J 1999;14:347–51. 10.1183/09031936.99.14234799 [DOI] [PubMed] [Google Scholar]
- 165.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 2002;62:2169–83. 10.2165/00003495-200262150-00001 [DOI] [PubMed] [Google Scholar]
- 166.Van Bambeke F, Tulkens PM. Macrolides: pharmacokinetics and pharmacodynamics. Int J Antimicrob Agents 2001;18(Suppl 1):S17–23. 10.1016/S0924-8579(01)00406-X [DOI] [PubMed] [Google Scholar]
- 167.Zobell JT, Young DC, Waters CD, et al. . Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations: I. aztreonam and carbapenems. Pediatr Pulmonol 2012;47:1147–58. 10.1002/ppul.22655 [DOI] [PubMed] [Google Scholar]
- 168.Zobell JT, Waters CD, Young DC, et al. . Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations: II. cephalosporins and penicillins. Pediatr Pulmonol 2013;48:107–22. 10.1002/ppul.22669 [DOI] [PubMed] [Google Scholar]
- 169.Stockmann C, Sherwin CM, Zobell JT, et al. . Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations: III. fluoroquinolones. Pediatr Pulmonol 2013;48:211–20. 10.1002/ppul.22667 [DOI] [PubMed] [Google Scholar]
- 170.Young DC, Zobell JT, Waters CD, et al. . Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations: IV. colistimethate sodium. Pediatr Pulmonol 2013;48:1–7. 10.1002/ppul.22664 [DOI] [PubMed] [Google Scholar]
- 171.Koh WJ, Jeong BH, Jeon K, et al. . Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012;186:797–802. 10.1164/rccm.201206-1088OC [DOI] [PubMed] [Google Scholar]
- 172.Hurley MN, Forrester DL, Smyth AR. Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis. Cochrane Database Syst Rev 2010;(10):CD008037 10.1002/14651858.CD008037.pub2 [DOI] [PubMed] [Google Scholar]
- 173.Clancy JP, Dupont L, Konstan MW, et al. . Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax 2013;68:818–25. 10.1136/thoraxjnl-2012-202230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Haverkamp MH, van Dissel JT, Holland SM. Human host genetic factors in nontuberculous mycobacterial infection: lessons from single gene disorders affecting innate and adaptive immunity and lessons from molecular defects in interferon-gamma-dependent signaling. Microbes Infect 2006;8:1157–66. 10.1016/j.micinf.2005.10.029 [DOI] [PubMed] [Google Scholar]
- 175.Browne SK, Holland SM. Immunodeficiency secondary to anticytokine autoantibodies. Curr Opin Allergy Clin Immunol 2010;10:534–41. 10.1097/ACI.0b013e3283402b41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chan ED, Bai X, Kartalija M, et al. . Host immune response to rapidly growing mycobacteria, an emerging cause of chronic lung disease. Am J Respir Cell Mol Biol 2010;43:387–93. 10.1165/rcmb.2009-0276TR [DOI] [PubMed] [Google Scholar]
- 177.Holland SM, Eisenstein EM, Kuhns DB, et al. . Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med 1994;330:1348–55. 10.1056/NEJM199405123301904 [DOI] [PubMed] [Google Scholar]
- 178.Milanés-Virelles MT, García-García I, Santos-Herrera Y, et al. . Adjuvant interferon gamma in patients with pulmonary atypical Mycobacteriosis: a randomized, double-blind, placebo-controlled study. BMC Infect Dis 2008;8:17 10.1186/1471-2334-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Martineau AR, Wilkinson KA, Newton SM, et al. . IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol 2007;178:7190–8. 10.4049/jimmunol.178.11.7190 [DOI] [PubMed] [Google Scholar]
- 180.Yuk JM, Shin DM, Lee HM, et al. . Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009;6:231–43. 10.1016/j.chom.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 181.Chan TY. Seasonal variations in vitamin-D status and the incidence of tuberculosis in different countries. Respiration 1999;66:196 doi:29369 [DOI] [PubMed] [Google Scholar]
- 182.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol 2008;37:113–19. 10.1093/ije/dym247 [DOI] [PubMed] [Google Scholar]
- 183.Martineau AR. Old wine in new bottles: vitamin D in the treatment and prevention of tuberculosis. Proc Nutr Soc 2012;71:84–9. 10.1017/S0029665111003326 [DOI] [PubMed] [Google Scholar]
- 184.Rolla M, D'Andrilli A, Rendina EA, et al. . Cystic fibrosis and the thoracic surgeon. Eur J Cardiothorac Surg 2011;39:716–25. 10.1016/j.ejcts.2010.07.024 [DOI] [PubMed] [Google Scholar]
- 185.Orens JB, Estenne M, Arcasoy S, et al. . International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745–55. 10.1016/j.healun.2006.03.011 [DOI] [PubMed] [Google Scholar]
- 186.Gilljam M, Scherstén H, Silverborn M, et al. . Lung transplantation in patients with cystic fibrosis and Mycobacterium abscessus infection. J Cyst Fibros 2010; 9:272–6. 10.1016/j.jcf.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 187.Lobo LJ, Chang LC, Esther CR Jr, et al. . Lung transplant outcomes in cystic fibrosis patients with pre-operative Mycobacterium abscessus respiratory infections. Clin Transplant 2013;27:523–9. 10.1111/ctr.12140 [DOI] [PubMed] [Google Scholar]
- 188.Huang HC, Weigt SS, Derhovanessian A, et al. . Non-tuberculous mycobacterium infection after lung transplantation is associated with increased mortality. J Heart Lung Transplant 2011;30:790–8. 10.1016/j.healun.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Knoll BM, Kappagoda S, Gill RR, et al. . Non-tuberculous mycobacterial infection among lung transplant recipients: a 15-year cohort study. Transpl Infect Dis 2012;14:452–60. 10.1111/j.1399-3062.2012.00753.x [DOI] [PubMed] [Google Scholar]
- 190.Chernenko SM, Humar A, Hutcheon M, et al. . Mycobacterium abscessus infections in lung transplant recipients: the international experience. J Heart Lung Transplant 2006;25:1447–55. 10.1016/j.healun.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 191.Qvist T, Pressler T, Thomsen VO, et al. . Nontuberculous mycobacterial disease is not a contraindication to lung transplantation in patients with cystic fibrosis: a retrospective analysis in a Danish patient population. Transplant Proc 2013;45:342–5. 10.1016/j.transproceed.2012.02.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.