Figure 1.
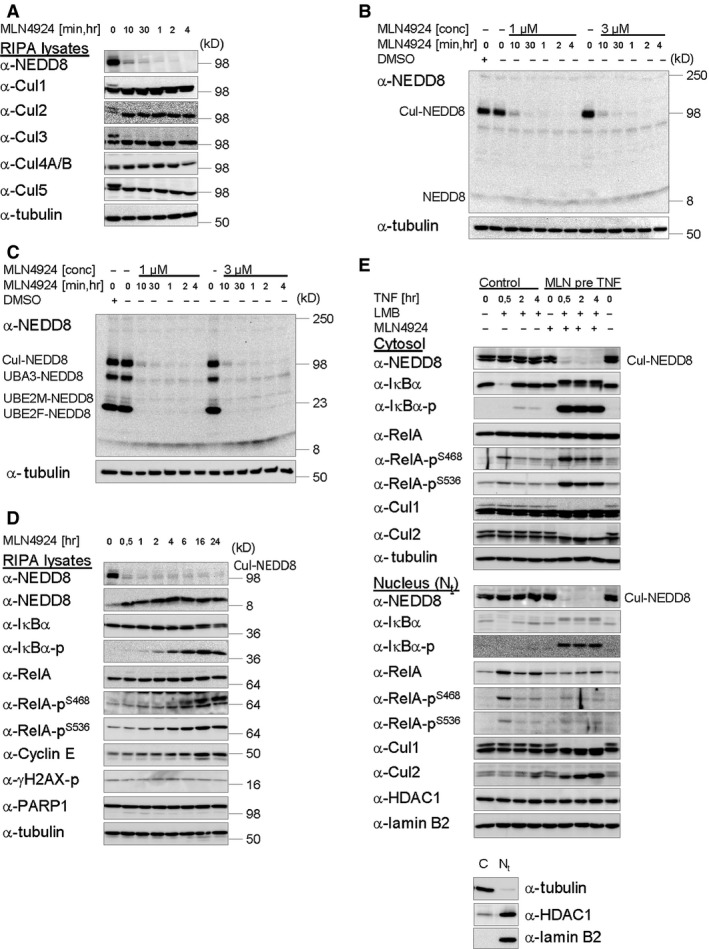
A functional neddylation pathway is essential for TNF‐induced degradation of IκBα and concomitant release and nuclear translocation of RelA. (A–C) Cullin neddylation is rapidly and efficiently inhibited by NAE inhibitor MLN4924. (D) Inhibition of cullin neddylation by MLN4924 in HeLa cells causes CRL substrate accumulation without induction of DNA damage or apoptosis. (A–D) After treatment with DMSO (vehicle) or MLN4924 (3 μM, or as indicated), cells were harvested by RIPA lysis at indicated times. Prior to analysis, samples were heat‐denatured by boiling in either the presence (A, B, D, E) or the absence (C) of β‐mercaptoethanol. (E) MLN4924 efficiently inhibits degradation of IκBα and RelA nuclear translocation in response to TNF. Cells treated with MLN4924 (3 μM) 10 min. prior to TNF (10 ng/ml) stimulation were harvested by subcellular fractionation at indicated times. Leptomycin B (LMB, 10 ng/ml) was added 15 min. after TNF stimulation to prevent Crm1‐dependent nuclear export of RelA. (A–E) Samples, as indicated, were analysed by IB. Detection of Tubulin (RIPA lysates and cytosol) or HDAC1 and Lamin B2 (total nuclear fractions, Nt) was performed for control of fractionation success (E) and equal protein load. Lacking accumulation of (i) H2AX phosphorylated at Ser139 (γH2AX) and (ii) PARP1 cleavage upon MLN4924 treatment (D) indicate the absence of DNA damage and induction of apoptosis respectively.
