Figure 1.
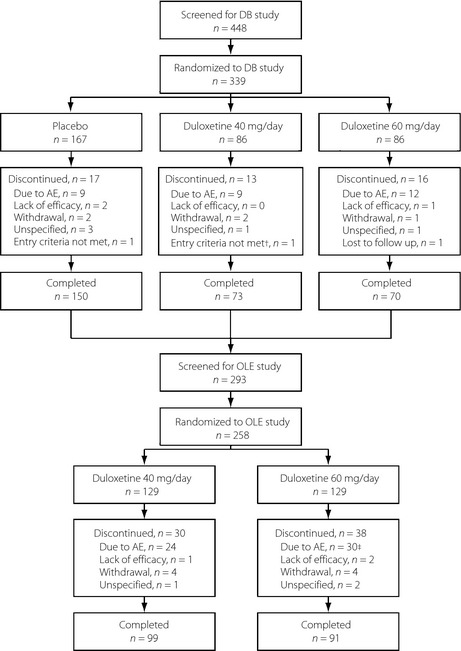
Participant flow diagram for the double‐blind (DB) and open‐label extension (OLE) studies. †One participant did not receive any study drug and was not assessed at any study visits after the start of the double‐blind study; this participant was excluded from the analysis. ‡One participant discontinued during the taper phase, not the treatment phase. AE, adverse event.
