Figure 3.
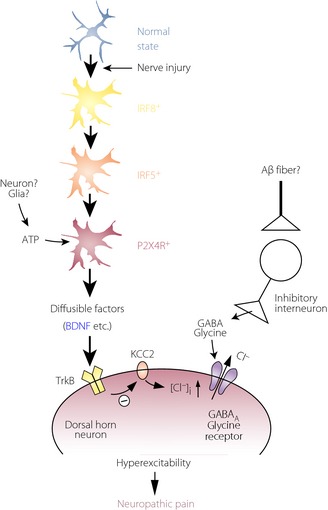
Schematic illustration for shifting spinal microglia toward a P2X4 receptor (P2XR4)‐expressing reactive state through an interferon regulatory factor 8 (IRF8)–IRF5 transcriptional axis after nerve injury, and a potential mechanism by which P2X4+ microglia cause hyperexcitability in dorsal horn neurons and neuropathic pain. After nerve injury, activated microglia show increased expression of IRF8, which in turn leads to induction of IRF5 expression. IRF5 then induces P2X4R expression by directly binding to the promoter region of the P2rx4 gene. P2X4R is activated by extracellular adenosine triphosphate (ATP; which could be presumably released from neurons or glial cells) and, in turn, release bioactive diffusible factors, such as brain‐derived neurotrophic factor (BDNF). BDNF downregulates the potassium‐chloride transporter, KCC2, through tropomyosin‐related kinase B, causes an increase in intracellular [Cl−], and leads to the collapse of the transmembrane anion gradient in dorsal horn neurons, which in turn induces depolarization of these neurons after stimulation by γ‐aminobutyric acid and glycine (which might be released in response to stimulation of Aβ fiber). The resultant hyperexcitability in the dorsal horn pain network induced by microglial factors could be responsible for neuropathic pain. GABA, γ‐aminobutyric acid.
