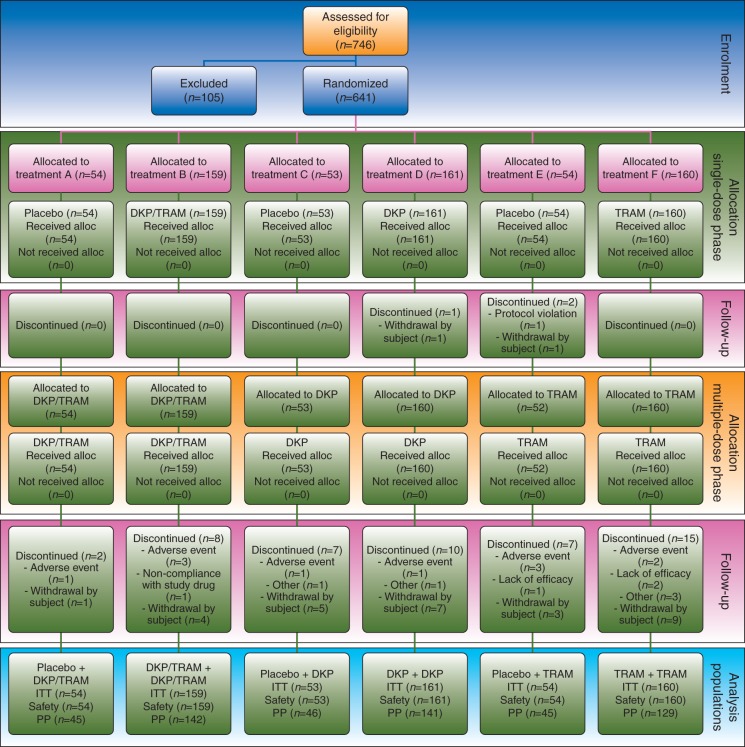
Fig 1.
Study CONSORT flow diagram. Participant flow, with the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome. Analysis populations were as follows: the ITT population included all patients randomized; the safety population included all patients randomized who received at least one dose of study treatment; and the PP population included all ITT patients with no major protocol violations. •, received at least one dose; alloc., allocated; DKP, dexketoprofen trometamol 25 mg; DKP/TRAM, dexketoprofen trometamol/tramadol hydrochloride 25 mg/75 mg; ITT, intention to treat; n, number of patients; PP, per protocol; TRAM, tramadol hydrochloride 100 mg.