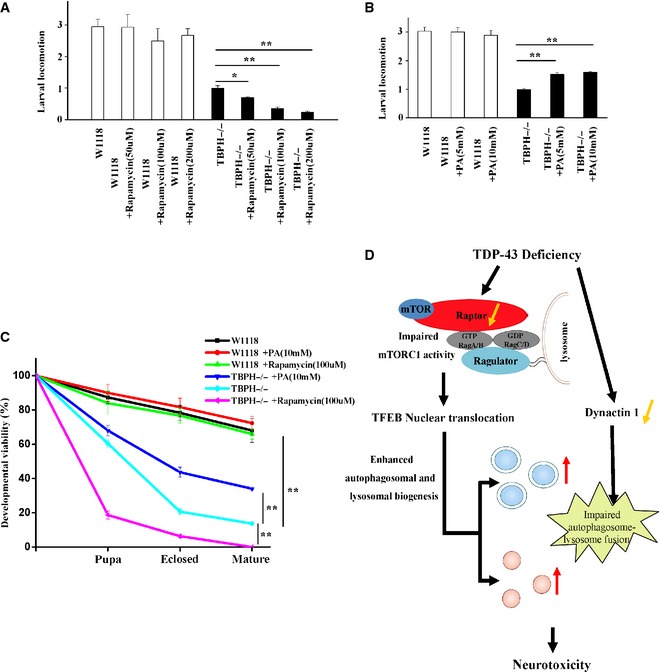
The first‐instar TBPH−/− larvae were treated with rapamycin for 72 h. Then, the third‐instar larvae of W1118 and TBPH−/− were subjected to larval locomotion assays. Data from three independent experiments represented as means ± S.E.M.; *, P < 0.05; **, P < 0.01; one‐way ANOVA.
Similar experiments as in (A) were performed, but using PA instead of rapamycin. Data are from three independent experiments, means ± S.E.M.; **, P < 0.01; one‐way ANOVA.
The first‐instar TBPH−/− larvae were selected to standard food containing rapamycin and PA. Quantitative developmental viability analysis of W1118, TBPH−/−, and TBPH−/− treated with rapamycin and PA. Data are from three independent experiments, means ± S.E.M.; **, P < 0.01; one‐way ANOVA.
A schematic model illustrates the molecular mechanism by which loss of TDP‐43 enhances TFEB‐mediated expression of ALP genes, blocks autophagosome–lysosome fusion, and induces neurodegeneration. TDP‐43 directly targets raptor, a key component of mTORC1, to regulate mTORC1–TFEB signaling. Meanwhile, TDP‐43 can affect the autophagosome–lysosome fusion in a dynactin 1‐dependent manner. The current study highlights the role of mTORC1 and dynactin 1 dysfunction, altered autophagy and lysosomal biogenesis in TDP‐43‐mediated neurotoxicity.