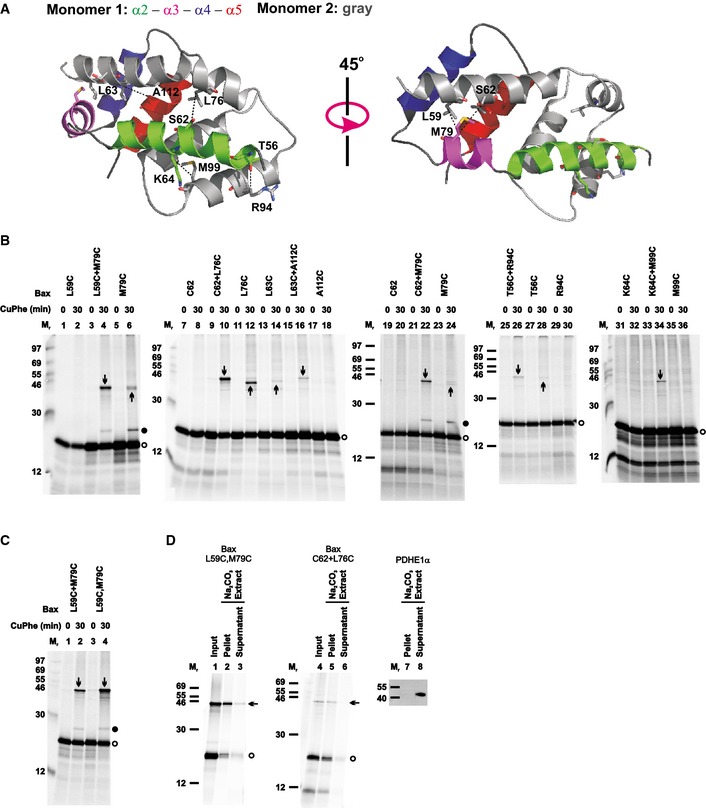
The crystal structure of the BH3‐in‐groove Bax homodimer (PDB entry 4BDU) is shown with one monomer colored gray and the other colored green, magenta, blue, and red for its α2, α3, α4, and α5 helices, respectively, as indicated. The residue pairs that were replaced with cysteine pairs in (B) are presented in stick form, and their β‐carbon atoms linked by dashed lines with the distances ranging from 5.0 to 6.0 Å.
The
in vitro synthesized [
35S]Met‐labeled single‐cysteine Bax proteins were activated and targeted to the mitochondria that were pretreated with NEM to block the sulfhydryls of mitochondrial proteins. The resulting mitochondria were isolated and oxidized by CuPhe for 30 min. NEM and EDTA were then added to stop the oxidation. For the “0 min” controls, NEM and EDTA were added before the addition of CuPhe. The resulting samples were analyzed by phosphorimaging after non‐reducing or reducing SDS–PAGE (see
Appendix Fig S2A).
Oxidized mitochondria with the radioactive single‐cysteine Bax protein pair or double‐cysteine Bax protein were prepared and analyzed as in (B).
Oxidized mitochondria with the radioactive single‐cysteine Bax protein pair or double‐cysteine Bax protein were prepared as in (B). After an aliquot was withdrawn as input, another aliquot was extracted by Na2CO3 (pH 11.5) and centrifuged through a sucrose cushion to separate the integral proteins in the membrane pellet from the soluble and peripheral proteins in the supernatant. The input, pellet, and supernatant were analyzed by non‐reducing SDS–PAGE and phosphorimaging. In a parallel control experiment, the pellet and supernatant were analyzed by reducing SDS–PAGE and immunoblotting with an antibody specific to PDHE1α, a soluble mitochondrial matrix protein.
Data information: In (B–D), protein standards are indicated on the side of phosphor images or immunoblots by their molecular mass (M
r). Open circles indicate Bax monomers. Upward arrows indicate disulfide‐linked dimers of the same single‐cysteine Bax mutant (e.g., M79C), and downward arrows disulfide‐linked dimers of two different single‐cysteine Bax mutants (e.g., L59C + M79C) or of the same double‐cysteine Bax mutant (L59C,M79C). Closed circles indicate the disulfide‐linked heterodimer of Bax M79C and the BH3 peptide (with a cysteine at position 62). The number of independent replicates done for each Bax mutant in (B–C),
n = 2 for C62+M79C, T56C, and R94C; 3 for L63C, L63C+A112C, A112C, K64C, K64C+M99C, and M99C; 4 for L59C+M79C, M79C, C62, C62+L76C, and T56C+R94C; 6 for L59C, and L76C; and 8 for L59C,M79C. For each Bax mutant and PDHE1α in (D),
n = 2.
Source data are available online for this figure.