Figure 4. G108E mutation in the groove disrupts the BH3‐in‐groove as well as the helices α2‐α3‐α4 Bax homodimer interface, but not tBid–Bax heterodimer interface.
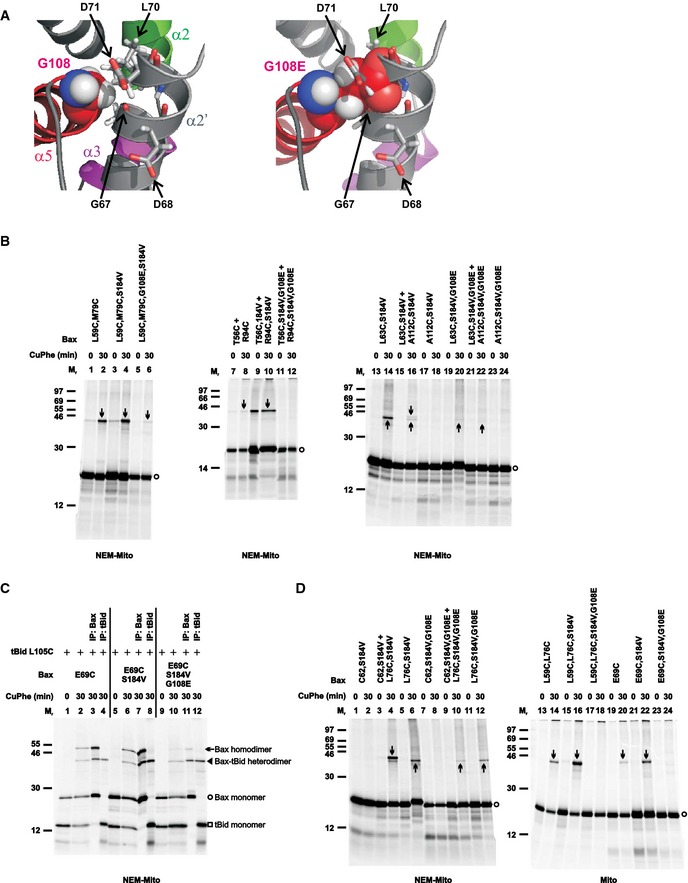
- Left panel, part of the BH3‐in‐groove dimer structure is shown with Gly108 from one monomer presented in sphere form, and Gly67 and Leu70 from the other monomer that have van der Waals' contacts with the Gly108 presented in stick form. Asp68 and Asp71 from the other monomer are also presented in stick form. Right panel, the same structure is shown with the Gly108 changed to glutamate (G108E), which results in steric clashes with the Gly67, Leu70, and Asp71, and electrostatic repulsions with the Asp68 and Asp71.
- The radioactive single‐ or double‐cysteine Bax proteins with or without the indicated mutations were activated and targeted to the mitochondria that were either untreated (mito) or pretreated with NEM (NEM‐mito), then oxidized and analyzed as in Fig 1B. Protein standards, and monomers and disulfide‐linked dimers of the Bax proteins are indicated as in Fig 1B. n = 2 for all mutants.
- The in vitro synthesized [35S]Met‐labeled Bax E69C protein with or without the additional mutations (G108E and/or S184V) were activated by the in vitro synthesized [35S]Met‐labeled tBid L105C protein and targeted to the Bax−/−/Bak−/− mitochondria that were pretreated with NEM. The resulting mitochondria were isolated and oxidized by CuPhe for 0 or 30 min. The resulting “0 min” samples (1 equivalent each) and “30 min” samples (1 equivalent each) were analyzed by non‐reducing SDS–PAGE and phosphorimaging. The remaining “30 min” samples (4 equivalent each) were immunoprecipitated (IP) by either Bax‐ or tBid‐specific antibody, and then analyzed by non‐reducing SDS–PAGE and phosphorimaging. The identities of the four major products, indicated on the right side of the image, were based on their Mr and recognition by the respective antibody. n = 2.
- The disulfide crosslinking data were obtained from the indicated Bax mutants and presented as in (B). n = 2 for all mutants.
Source data are available online for this figure.
