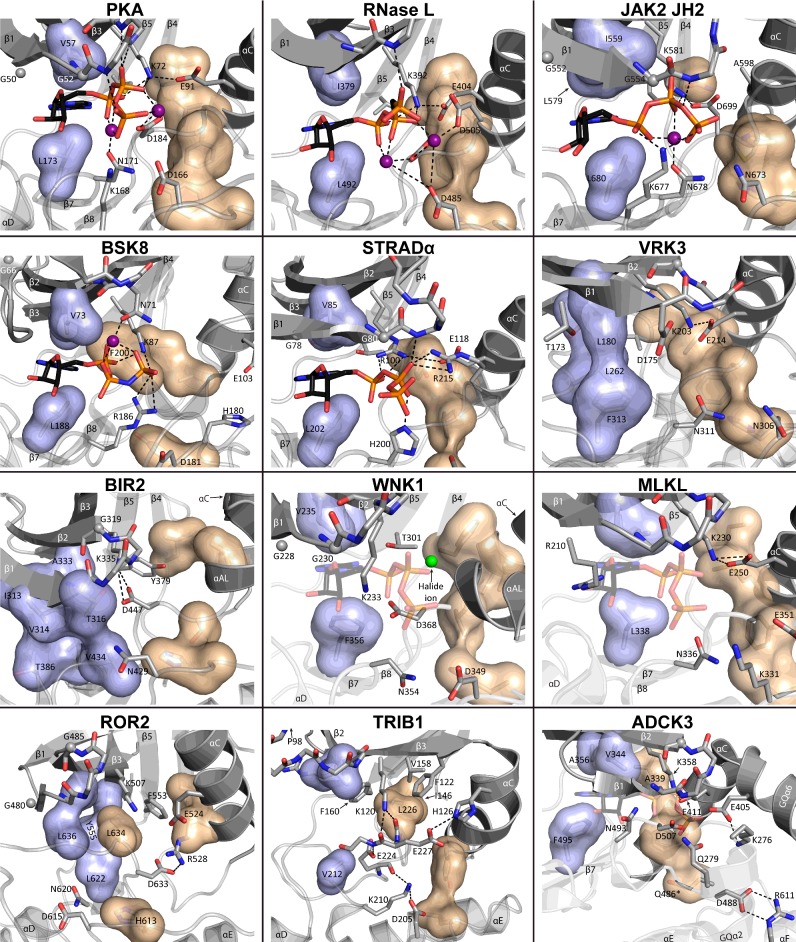
Figure 2. Diverse ATP-binding pockets and nt-binding modes among pseudokinases.
Crystal structures of ATP-binding pockets of selected representative (pseudo)kinases and PKL proteins with varying nt-binding modes. Shown are human PKA (PDB: 4WB5), human RNase L (4OAV), human JAK2 JH2 (4FVQ), Arabidopsis thaliana BSK8 (4I94), human STRADα (3GNI), human VRK3 (2JII), A. thaliana BIR2 (4L68), Rattus norvegicus WNK1 (4Q2A), human MLKL (4MWI), human ROR2 (4GT4), human TRIB1 (5CEM) and human ADCK3 (4PED). ATP shown in WNK1, MLKL and ADCK3 was modelled based on PKA (4WB5), as no ATP-bound structures exist, even though they verifiably bind adenine nts. ATP or ATP-analogues (e.g. AMP-PNP for BSK8) are shown as sticks with elements coloured as follows: carbon: black, oxygen: red, nitrogen: blue, phosphorus: orange. Divalent cations are shown as purple spheres. The halide ion in WNK1 is shown in green. The R spine is shown as a beige volume filling model, whereas the top of the C spine, encompassing the hydrophobic purine-binding pocket is shown in light blue. Hydrophobic side chains occluding the purine-binding pocket are shown as part of the C spine for VRK3, BIR2 and ROR2, where the pocket is occluded. Gly-rich loop glycines are shown as grey spheres with Gly-rich loop side chains omitted, unless of special note. Water molecules from the crystal structures have been omitted for clarity. *Only one possible conformation given for Gln486, ADCK3 is shown.