Abstract
Background
We have prospectively studied a three month course of clarithromycin (substituted by Prevpac®, lansoprazole/ amoxicillin/ clarithromycin, in the first two wks when stool H pylori+) for non-bulky, advanced stage indolent lymphoma. These patients are often candidates for expectant monitoring and it is during this period that a window of opportunity may exist to identify and treat associated infections.
Methods
All previously untreated patients with a new diagnosis of indolent lymphoma (FL and non-FL) meeting GELF criteria were treated with 12 weeks of clarithromycin. There were 32 evaluable patients, 4 of whom had stool H pylori.
Results
At one month post-antibiotic therapy, we have observed lymphoma responses in 7 of 32 patients (21.9%). Two additional patients had objective response during followup (28.1% overall response). The median treatment free survival for antibiotic responders is 69.9 months and for non-responders, 30.6 months (p = 0.019).
Conclusion
Three response patterns have been noted, perhaps suggestive of an immune-mediated response -- prompt PET negative; flair with delayed PET negative response; and gradual continuous improvement. This prospective study appears promising, may be a step toward developing a lymphoma prevention strategy by reducing “antigen drive,” and deserves further clinical/biological study. http://clinicaltrials.gov/show/NCT00461084
Keywords: Clarithromycin, indolent lymphoma, free survival, antibiotic, improvement, treatment
1 Introduction
While many new therapies have become available for indolent lymphomas, none have proven to be curative other than allotransplantation. The value of an allotransplant‘s new immunity in controlling indolent lymphoma is well elucidated in recent reviews, and the concept of a proliferative stimulus and a suppressive immune control are now well accepted [1-4]. Although the etiology of the proliferative stimulus is generally unknown, several infections (i.e., Helicobacter pylori in gastric mucosa-associated lymphoid tissue or MALT and hepatitis C virus (HCV) in HCV-associated lymphoma, do appear to provide the proliferative signal -- “antigen-drive” – and, as such, treatment of the underlying infection appears to lead to improvement/resolution of the lymphoma.
Expectant monitoring remains a standard initial strategy in the management of many indolent lymphomas, unless the lymphoma medically requires therapy or a known associated infectious agent is detected. With frequent screening for adenopathy, as is commonly done in the United States, the proportion of patients appropriate for expectant monitoring has risen (up to 60% in a recent Stanford report regarding follicular lymphoma) [5]. Objective criteria for selecting an expectant monitoring approach are currently available (Groupe d-Etude des Lymphomes Folliculaires -- GELF criteria in Table 1) [6].
Table 1.
GELF criteria for Expectant Monitoring
| Indolent lymphoma diagnosis |
|---|
| No evidence of histologic transformation |
| None of the following: |
| Nodal or extranodal mass with a diameter of > 7 cm |
| Involvement of at least 3 nodal sites (each with a diameter of > 3 cm) |
| Systemic symptoms |
| Symptomatic splenomegaly |
| Ureteral compression |
| Significant cytopenias |
We have previously reported that approximately one-third of patients with indolent lymphoma may have an identifiable and potentially treatable infection (H pylori, HCV, or small bowel bacterial overgrowth). Anecdotal responses to antibiotic therapy were noted in this report and in other similar studies [2,7,8]. Of interest are those patients in our prior report [7] who have had durable clinical remissions for 5+ years following antibiotics only.
Based on these preliminary results, we now report a prospective study of single agent clarithromycin administered for three months in non-bulky advanced stage, indolent lymphoma in an attempt to reduce “antigen drive”. All patients met GELF criteria to be monitored expectantly and all were tested for H pylori stool antigen (if positive, Prevpac® was administered and substituted for the first two weeks of planned clarithromycin).
2 Methods
Patient characteristics are listed in Table 2. Eligibility included Follicular Lymphoma (FL: grades 1, 2, or 3A) or non-FL histologies (nFL: small lymphocytic lymphoma, lymphoplasmacytoid lymphoma, marginal zone lymphoma, nodal and extranodal mucosa-associated lymphoid tissue -- MALT types). Pathology was confirmed at Memorial Hospital. All patients were previously untreated with non-bulky advanced stage (intraabdominal stage II, III, IV) disease which did not require initial therapy according to GELF criteria (See Table 1) [7].
Table 2.
Patient characteristics
| Characteristic | N | H. pylori positive |
|---|---|---|
| Evaluable patients | 32 | 4 |
| Median followup | 63.6 months (range, 24.5-77.6) |
|
| Median Age | 53.5 years (range, 36-81) |
|
| Gender M:F | 18:14 | |
| Histology | ||
| Follicular | 22 | 3 |
| Non-Follicular | 10 | 1 |
| Stage | ||
| II | 2 | |
| III | 16 | |
| IV | 14 |
Baseline staging included computed tomography (CT) scan of the torso, positron emission tomography (PET) scan, bone marrow biopsy and routine laboratory studies. H pylori stool antigen was tested in all patients.
Patients were 18 years old or greater, Karnofsky performance status greater than 70%, no prior radiation therapy, no evidence of histologic transformation, no history of Human immunodeficiency virus or prior malignancy within the prior five years. Patients with evidence of hepatitis B or C virus exposure or other known active infection were excluded. All patients provided Institutional Review Board voluntary written consent.
All eligible patients were treated with a 12-week course of clarithromycin, 500 mg by mouth twice daily. Those patients who tested positive for H pylori, received two weeks of lansoprazole/amoxicillin/clarithromycin (Prevpac®) followed by ten weeks of clarithromycin only. Restaging was performed within one month of completion of antibiotic therapy with both CT and PET scan imaging. Moreover, patients who were H pylori positive underwent repeat stool testing to document infection clearance within three months post-therapy. Thereafter, patients were monitored according to MSKCC guidelines for lymphoma progression or regression. Response, if any, was measured according to the International Working Group standard response criteria for non-Hodgkin’s lymphoma; including complete response (CR), complete response uncertain (CRu) and partial response (PR) [9].
This was a prospective clinical trial. Descriptive statistics were utilized to report response to antibiotic therapy and patient demographics. Actuarial treatment-free survival was measured from end of antibiotic therapy to initiation of standard lymphoma therapy or last followup. The Kaplan Meier curves were created using SPSS version 21.[10] The protocol primary trial data was reviewed and analyzed by all authors; and the clinical trial was registered at, http://clinicaltrials.gov/show/NCT00461084.
3 Results
Between September 2007 and February 2012, there were 32 evaluable patients enrolled including 14 females and 18 males. Median age was 53.5 years (range 36-81). The median follow-up of surviving patients is 63.6 months (range 24.5-77.6). FL was diagnosed in 22; nFL in 10. Disease distribution included: Stage II, 2; Stage III, 16; and Stage IV, 14. H. pylori stool antigen was positive in four: 3 with FL and 1 with nFL. (See Table 2)
We have observed lymphoma responses in 7 of 32 patients (21.9%) at one month post-antibiotic therapy. Two additional patients have had objective response (9 or 28.1% overall), at 18.7 and 48.3 months post-antibiotic therapy.
Among 22 patients with FL, eight have required treatment, three of whom developed histologic transformation at first progression (including one who was an antibiotic responder). Fourteen patients have not needed institution of standard lymphoma therapy, including 4 of 6 antibiotic responders.
Among ten patients with nFL, three have required treatment and none had histologic transformation at that time. The three patients with objective response all remain free of standard lymphoma therapy.
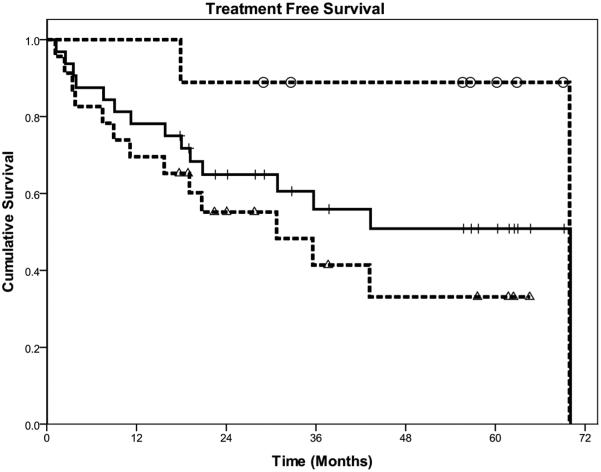
The median treatment-free survival for all patients is 69.9 months (See Figure 1). For FL, the median treatment-free survival is 69.9 months; and for non-FL, the median survival has not been reached with 54.9% remaining treatment-free at the median follow-up of 63.6 months.
Figure 1.
Actuarial Treatment Free Survival from end of antibiotic therapy. Nine responders to Ab (-0--); all 32 patients (--); and 23 non-responders to Ab (-Δ--).
For responders to clarithromycin, 89% remain free of standard treatment at 63.6 months. One patient has progressed at 69.9 months, resulting in a median treatment-free survival for responders of 69.9 months; non-responders have a median treatment-free survival of 30.6 months (Log rank p-value, 0.019).
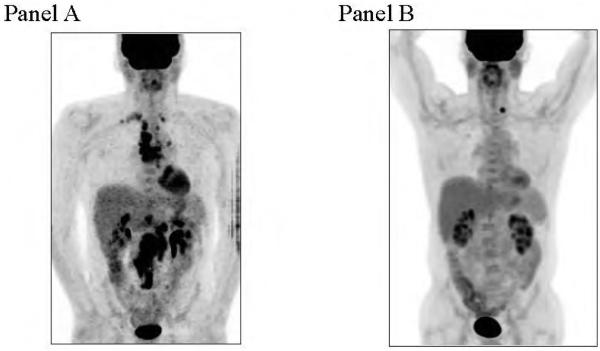
We have observed three patterns of response in our nine responding patients: prompt PET negative response; flair followed by delayed response (See Figure 2); and a gradual but continuous improvement/response.
Figure 2.
PET scan in FL patient with H pylori. Mild flare at 6 months (panel A); and negative PET at 18 months (Left thyroid nodule noted; panel B).
Following antibiotic therapy, at least two clinical flairs occurred with later improvement: one patient with FL (H pylori negative) developed a rapidly enlarging mass seven months after clarithromycin completion and excisional biopsy revealed only necrotic B cells. This patient has been clinically well thereafter for 60+ months. Another patient with FL (H pylori positive) was stable at post-Prevpac® / clarithromycin restaging, went on to a mild tumor flare at six months, and at 18 months after antibiotic therapy, achieved a PET negative response. (See Figure 2).
The most common side effect of clarithromycin was metallic taste. This did not interfere with planned treatment. One patient experienced a drug eruption after nine days and was removed from the study.
4 Discussion
The antigen drive association of gastric MALT lymphoma, an indolent B cell lymphoma, with H pylori is well recognized [2,8]. We have previously studied this and other possible associated infections in patients with non-bulky, advanced stage indolent lymphoma [7]. We reported an incidence of 24.5% for H. pylori (13 of 53); 6.1% for HCV (3 of 49); and 20.4% for small bowel bacterial overgrowth -- SBBO (11 of 54) as detected by breath test. Those with H. pylori received a two-week course of Prevpac® (as in this study), but no additional follow-on clarithromycin. One patient with FL became PET negative after Prevpac® therapy and has remained in remission for 10+ years. Two nFL patients achieved responses which have been similarly durable after successful HCV antiviral therapy. Among 11 patients with SBBO, two have not had clinical progression for 6.5+ years (nFL) and 9+ years (FL) following two weeks of ciprofloxacin.
We now report a prospective study utilizing a longer antibacterial therapy course in a similar patient population. Patients were not tested for SBBO in the current study and those with hepatitis B and C viral infection were excluded from the trial. All eligible patients received 12 weeks of clarithromycin therapy, with Prevpac® substituted for the first two weeks in those who were H. pylori stool antigen positive.
Clarithromycin was selected for use in this study because of its antibacterial activity and its successful use in other lymphoma trials [2,8]. Macrolide antibiotics are also considered immune modulators, but these properties have not been investigated in lymphoma [11].
Although this is a small study, we have observed gratifying responses with sustained durability. Seven of 32 (21.9%) patients had an objective response to antibiotic therapy at the one month restaging. With continued follow-up, two additional patients had objective responses at 18.7 and 48.3 months post-antibiotic therapy. Moreover, for those patients with objective response to clarithromycin, the treatment-free survival is 89.9% at 63.6 months with one late progression at 69.9 months. Whereas for non-responders, the median treatment-free survival is 30.6 months (Log rank p-value, 0.019, Figure 1).
This result appears to compare favorably with prior studies examining the role of expectant monitoring. For example, Brice et al. [12] reported a 24 month median freedom from treatment in low tumor burden follicular lymphoma. This study reveals a median treatment-free survival for low tumor burden follicular lymphoma of 69.9 months.
There were four of 32 (12.5%) who were H pylori positive, and two of these, both with FL, have achieved PET CRs. One patient was PET negative one month following antibiotic therapy alone and remained in remission for 69.9 months post-antibiotic therapy and was H pylori negative at progression. One patient was stable at post-antibiotic restaging, went on to a mild tumor flare at six months, but at 18 months, has achieved a PET negative response (See Figure 2). The other two patients who were H pylori positive did not have a lymphoma response: one FL with stable findings for six months, then developed a possible flair, and was initiated on standard systemic therapy; one nFL had a resistant H pylori organism and was taken off study.
In this prospective study, there were two documented clinical flairs followed by later improvement: one FL patient (H pylori negative) developed a rapidly enlarging mass seven months after antibiotic therapy and an excisional biopsy revealed only necrotic B cells. This patient has been clinically well thereafter for 60+ months since antibiotic therapy. One FL patient (H pylori positive) was stable at post-antibiotic restaging, went on to a mild tumor flare at six months, and at 18 months after antibiotic therapy, achieved a PET negative response (See Figure 2).
In summary, we have observed nine objective responses to clarithromycin antibiotic therapy with three clinical patterns suggesting an immune-mediated mechanism: a prompt PET negative response; a flair followed by a delayed PET negative response; and a gradual continuous improvement/delayed response. These patterns are suggestive of the immune-related responses outlined by Wolchok et al. [13] for immune therapy of solid tumors.
Although this is a small study, 9 of 32 (28.1%) patients have had objective responses and for those patients responding to antibiotics, the treatment-free survival is 89.9% at 60 months with one late progression at 69.9 months. We are encouraged by this result and conclude that this prospective study may be a step toward developing a lymphoma preventive strategy by reducing “antigen drive”. Clinical, biologic and immune correlates are required to better understand the apparent immune responses seen.
Acknowledgements
Support: Memorial Sloan Kettering Cancer Center Philanthropic Funds Thanks to Ernestine Jacobs for her excellent assistance in manuscript preparation
Footnotes
Conflict of interest: The authors declare there are no competing financial interests.
Authorship Contribution: Study design: CSP, PAH, JG, AN, MLP, SC, GP, AJM. Treated patients and collected clinical data: CSP. PAH, JG, AN, MLP, SC. Data analysis and interpretation: CSP, PAH, JG, AN, MLP, JW, JM, OD, GP, AJM. All authors wrote, revised and approved the final manuscript.
Contributor Information
Carol S Portlock, Department of Medicine, Lymphoma Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Paul A Hamlin, Memorial Sloan Kettering Cancer Center, Department of Medicine, 1275 York Avenue New York, NY, 10065 USA.
John F Gerecitano, Memorial Sloan Kettering Cancer Center, Department of Medicine, 1275 York Avenue New York, NY, 10065 USA.
Ariela Noy, Memorial Sloan Kettering Cancer Center, Department of Medicine, 1275 York Avenue New York, NY, 10065 USA.
Maria Lia Palomba, Memorial Sloan Kettering Cancer Center, Department of Medicine, 1275 York Avenue New York, NY, 10065 USA.
Janelle Walkley, Memorial Sloan Kettering Cancer Center, Department of Medicine, 1275 York Avenue New York, NY, 10065 USA.
Stacie Corcoran, Memorial Sloan Kettering Cancer Center, Office of Physician-in-Chief, 1275 York Avenue New York, NY, 10065 USA.
Jocelyn Migliacci, Memorial Sloan Kettering Cancer Center, Department of Medicine, 1275 York Avenue New York, NY, 10065 USA.
Heiko Schoder, Memorial Sloan Kettering Cancer Center, Department of Radiology, 1275 York Avenue New York, NY, 10065 USA.
Genovefa Papanicolaou, Memorial Sloan Kettering Cancer Center, Department of Medicine, 1275 York Avenue New York, NY, 10065 USA.
Arnold J Markowitz, Memorial Sloan Kettering Cancer Center, Department of Medicine, 1275 York Avenue New York, NY, 10065 USA.
References
- [1].Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Semin. Cancer Biol. 2013;23:410–21. doi: 10.1016/j.semcancer.2013.09.001. DOI: 10.1016/j.semcancer.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferreri AJ, Govi S, Ponzoni M. Marginal zone lymphomas and infectious agents. Semin. Cancer Biol. 2013;23:431–40. doi: 10.1016/j.semcancer.2013.09.004. DOI: 10.1016/j.semcancer.2013.09.004. [DOI] [PubMed] [Google Scholar]
- [3].Amé-Thomas P, Tarte K. The yin and the yang of follicular lymphoma cell niches: Role of microenvironment heterogeneity and plasticity. Semin. Cancer Biol. 2014;23:23–32. doi: 10.1016/j.semcancer.2013.08.001. pii: S1044-579X(13)00077-1, DOI: 10.1016/j. semcancer.2013.08.001. [DOI] [PubMed] [Google Scholar]
- [4].Khouri IF, Champlin RE. Nonmyeloablative allogeneic stem cell transplantation for non-Hodgkin lymphoma. Cancer J. 2012;18:457–62. doi: 10.1097/PPO.0b013e31826b124c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122:981–87. doi: 10.1182/blood-2013-03-491514. DOI: 10.1182/blood-2013-03-491514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Solal-Celigny P, Lepage E, Brousse N, et al. Recombinant interferon alfa-2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. Groupe d’Etude des Lymphomes de l’Adulte. N. Engl. J. Med. 1993;329:1608–14. doi: 10.1056/NEJM199311253292203. [DOI] [PubMed] [Google Scholar]
- [7].Portlock CS, Hamlin P, Noy A, et al. Infectious disease associations in advanced stage, indolent lymphoma (follicular and nonfollicular): developing a lymphoma prevention strategy. Ann. Oncol. 2008;19:254–8. doi: 10.1093/annonc/mdm484. [DOI] [PubMed] [Google Scholar]
- [8].Kuo SH, Cheng AL. Helicobacter pylori and mucosa-associated lymphoid tissue: what’s new. Hematology Am. Soc. Hematol. Educ. Program. 2013:109–17. doi: 10.1182/asheducation-2013.1.109. DOI: 10.1182/asheducation-2013.1.109. [DOI] [PubMed] [Google Scholar]
- [9].IBM Corp . IBM SPSS Statistics for Windows. Armonk, NY: 2012. Version 21.0. [Google Scholar]
- [10].Cheson B, Horning SJ, Coiffer B, et al. Report of an international workshop to standardized response criteria for non-Hodgkin’s lymphomas. J. Clin. Oncol. 1999;17:1244–53. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- [11].Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 1997;15:1110–17. doi: 10.1200/JCO.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- [13].Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. DOI: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]