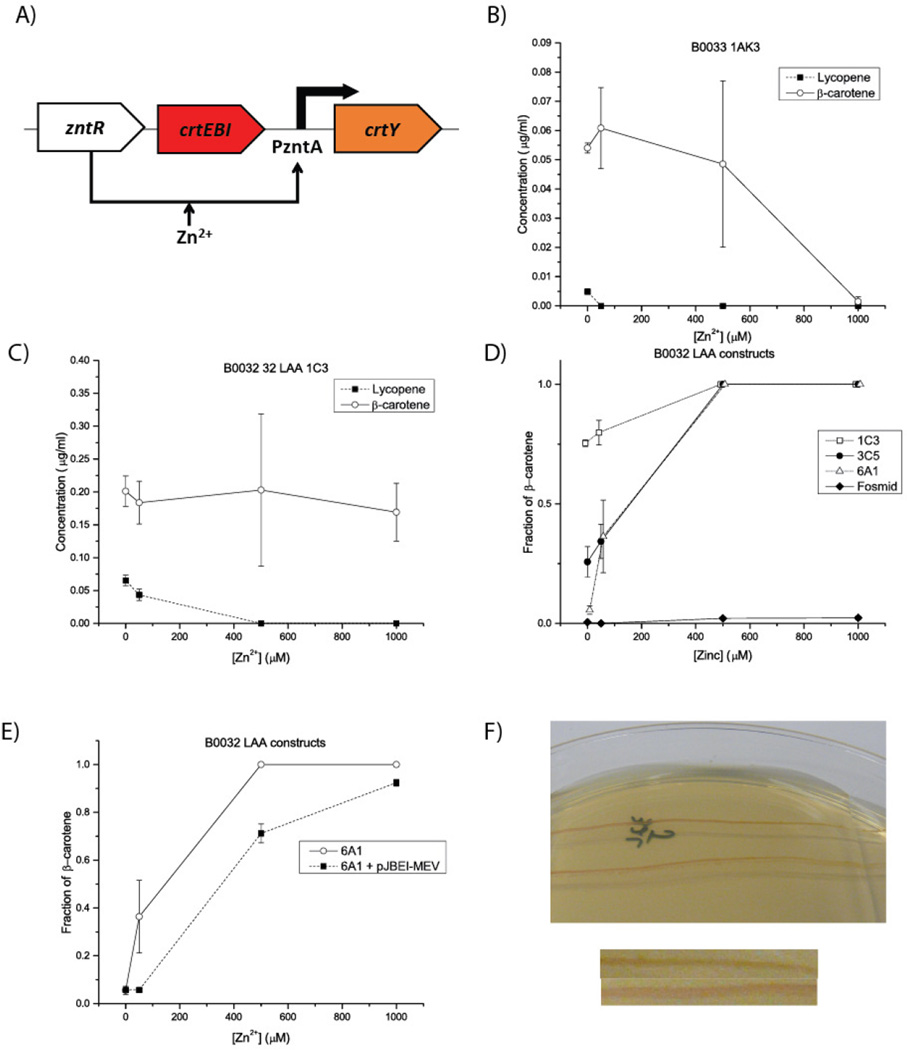
Figure 4.
Varying regulatory sequences and vector enables a lycopene-dominated state and allows manipulation of the concentration at which it disappears. All error bars represent the standard error of the mean. All measurements were taken at 0, 50, 500, or 1000 µM zinc, though an offset has been applied in panel D for visualization purposes. A) A simplified version of the construct used to test only carotenoid production for a selection of constructs with varied regulatory sequences and vectors. Ribosomal binding sites and degradation tags are omitted for simplicity. B) Changing only to a very weak ribosomal binding site is insufficient to produce a lycopene-only state, and generally insufficient to produce any lycopene at all. This suggests that multiple levels of control must be used. C) Using a moderate ribosomal binding site and a strong degradation tag (LAA) enables the detectable production of lycopene but still does not enable a lycopene-dominated state. D) Changing the vector carrying the construct from panel C enables the presence of a lycopene-only state. For the lowest-copy plasmid (the fosmid), so little CrtY is accumulated even at full zinc induction that only a lycopene-dominated state can be observed. The low-copy 6A1 vector offers two distinct states. E) Co-transforming with a plasmid containing the mevalonate pathway (pJBEI-MEV) to supplement production of lycopene alters the transition point between the lycopene and β-carotene states. At 50 µM Zn2+, the pJBEI-MEV-supplemented cells have still produced orders of magnitude more lycopene than β-carotene, while the non-supplemented strain is already transitioning to a β-carotene state. Of note is that the lycopene production at no supplemented zinc is three times higher in the supplemented strain, as expected. F) Streak of pJBEI-MEV-supplemented strain from panel E on zinc gradient plates showing a fairly distinct transition between states. The cells change from red to orange going left to right. Below the plates are two parts of the same image cropped next to each other to highlight the color differences.