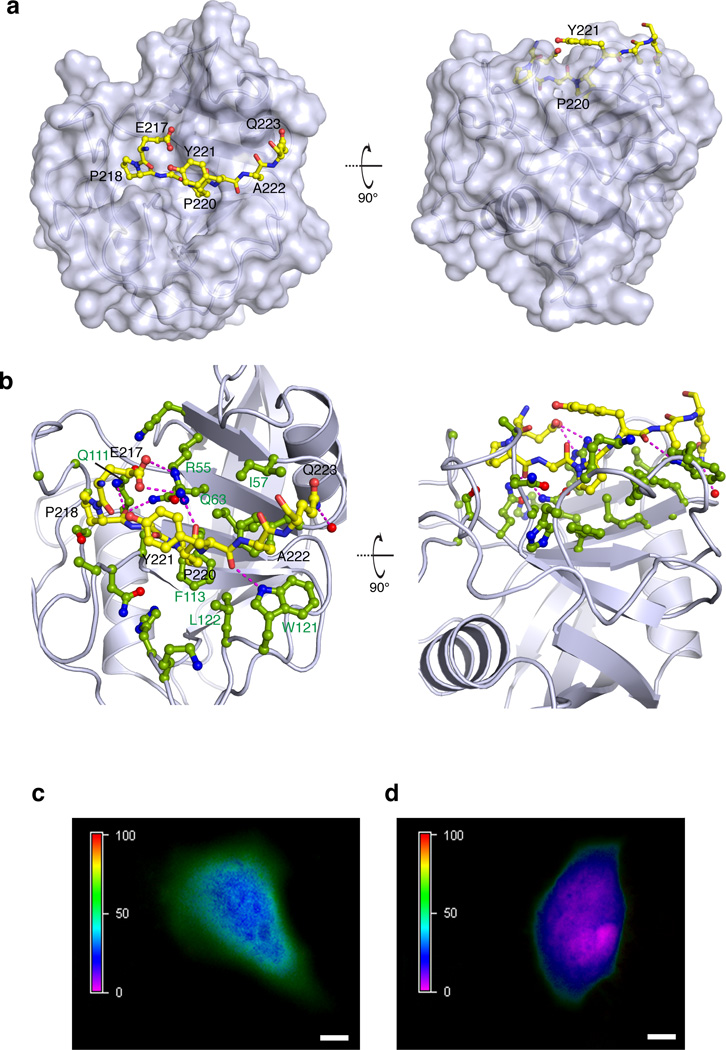
Figure 2. Specific interaction between CypA and CrkII in vitro and in vivo.
(a) Structure of CypA in complex with the CrkII peptide consisting of residues Ser216-Pro225. CypA is shown in a solvent-accessible surface representation whereas CrkII is shown as a ball-and-stick model. CrkII residues Glu217-Gln223 are shown (CrkII residues Pro216, Pro224 and Pro225 do not interact with CypA). The backbone of the CrkII region interacting with CypA is buried inside the catalytic cleft of CypA. Two views related by a 90° rotation about the x axis are shown. CrkII is in the trans conformation. (b) Interacting interface between CypA and CrkII. CypA is shown in light blue ribbon and the side chains of the residues interacting with CrkII are shown in ball-and-stick (green). CrkII is shown in yellow ball-and-stick. The magenta lines denote inter-molecular polar contacts (hydrogen bonds and/or salt bridges). (c) FRET analysis of HeLa cells transiently co-transfected with CrkII-YFP and CypA-CFP. The FRET efficiency is color coded from 0–100% on the left bar. Images were acquired on live cells after confirming comparable expression of both proteins. An average FRET efficiency of 70% is observed between the two fluorophores. Scale bar: 10 µm. (d) FRET analysis of HeLa cells transiently co-transfected with CrkII-YFP and CypA-CFP and treated with 25 µM of CsA before imaging. The average FRET efficiency drops to ~15% indicating that the complex is disrupted in the cell. Scale bar: 10 µm.